Trametinib overcomes KRAS-G12V-induced osimertinib resistance in a leptomeningeal carcinomatosis model of EGFR-mutant lung cancer
- PMID: 34145930
- PMCID: PMC8409422
- DOI: 10.1111/cas.15035
Trametinib overcomes KRAS-G12V-induced osimertinib resistance in a leptomeningeal carcinomatosis model of EGFR-mutant lung cancer
Abstract
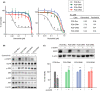
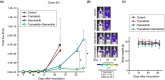
Leptomeningeal carcinomatosis (LMC) occurs frequently in non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutations and is associated with acquired resistance to EGFR tyrosine kinase inhibitors (EGFR-TKIs). However, the mechanism by which LMC acquires resistance to osimertinib, a third-generation EGFR-TKI, is unclear. In this study, we elucidated the resistance mechanism and searched for a novel therapeutic strategy. We induced osimertinib resistance in a mouse model of LMC using an EGFR-mutant NSCLC cell line (PC9) via continuous oral osimertinib treatment and administration of established resistant cells and examined the resistance mechanism using next-generation sequencing. We detected the Kirsten rat sarcoma (KRAS)-G12V mutation in resistant cells, which retained the EGFR exon 19 deletion. Experiments involving KRAS knockdown in resistant cells and KRAS-G12V overexpression in parental cells revealed the involvement of KRAS-G12V in osimertinib resistance. Cotreatment with trametinib (a MEK inhibitor) and osimertinib resensitized the cells to osimertinib. Furthermore, in the mouse model of LMC with resistant cells, combined osimertinib and trametinib treatment successfully controlled LMC progression. These findings suggest a potential novel therapy against KRAS-G12V-harboring osimertinib-resistant LMC in EGFR-mutant NSCLC.
Keywords: epidermal growth factor receptor; leptomeningeal carcinomatosis; non-small cell lung cancer; osimertinib; trametinib.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Seiji Yano received speaking fees from Pfizer and Chugai Pharmaceutical Co., Ltd., as well as a research grant from Chugai Pharmaceutical Co., Ltd. The other authors have nothing to disclose.
Figures




References
-
- Barnholtz‐Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865‐2872. - PubMed
-
- Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non‐small‐cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol. 2015;20:674‐679. - PubMed
-
- Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11:1962‐1969. - PubMed
-
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2012;13:239‐246. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

