Tunicates Illuminate the Enigmatic Evolution of Chordate Metallothioneins by Gene Gains and Losses, Independent Modular Expansions, and Functional Convergences
- PMID: 34146103
- PMCID: PMC8476144
- DOI: 10.1093/molbev/msab184
Tunicates Illuminate the Enigmatic Evolution of Chordate Metallothioneins by Gene Gains and Losses, Independent Modular Expansions, and Functional Convergences
Abstract
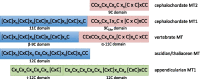
To investigate novel patterns and processes of protein evolution, we have focused in the metallothioneins (MTs), a singular group of metal-binding, cysteine-rich proteins that, due to their high degree of sequence diversity, still represents a "black hole" in Evolutionary Biology. We have identified and analyzed more than 160 new MTs in nonvertebrate chordates (especially in 37 species of ascidians, 4 thaliaceans, and 3 appendicularians) showing that prototypic tunicate MTs are mono-modular proteins with a pervasive preference for cadmium ions, whereas vertebrate and cephalochordate MTs are bimodular proteins with diverse metal preferences. These structural and functional differences imply a complex evolutionary history of chordate MTs-including de novo emergence of genes and domains, processes of convergent evolution, events of gene gains and losses, and recurrent amplifications of functional domains-that would stand for an unprecedented case in the field of protein evolution.
Keywords: Chordata; Tunicata; ascidians/thaliaceans/appendicularians; metallothionein domains; metallothionein evolution; modular proteins.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures





Similar articles
-
Modularity in Protein Evolution: Modular Organization and De Novo Domain Evolution in Mollusk Metallothioneins.Mol Biol Evol. 2021 Jan 23;38(2):424-436. doi: 10.1093/molbev/msaa230. Mol Biol Evol. 2021. PMID: 32915992 Free PMC article.
-
Metallothioneins of the urochordate Oikopleura dioica have Cys-rich tandem repeats, large size and cadmium-binding preference.Metallomics. 2018 Nov 14;10(11):1585-1594. doi: 10.1039/c8mt00177d. Metallomics. 2018. PMID: 30284576
-
Metal dealing at the origin of the Chordata phylum: the metallothionein system and metal overload response in amphioxus.PLoS One. 2012;7(8):e43299. doi: 10.1371/journal.pone.0043299. Epub 2012 Aug 14. PLoS One. 2012. PMID: 22905252 Free PMC article.
-
Regulatory elements retained during chordate evolution: coming across tunicates.Genesis. 2015 Jan;53(1):66-81. doi: 10.1002/dvg.22838. Epub 2014 Dec 1. Genesis. 2015. PMID: 25394183 Review.
-
Transitional chordates and vertebrate origins: Tunicates.Curr Top Dev Biol. 2021;141:149-171. doi: 10.1016/bs.ctdb.2020.10.001. Epub 2020 Dec 16. Curr Top Dev Biol. 2021. PMID: 33602487 Review.
Cited by
-
Metals and metallothionein evolution in snails: a contribution to the concept of metal-specific functionality from an animal model group.Biometals. 2024 Jun;37(3):671-696. doi: 10.1007/s10534-024-00584-3. Epub 2024 Feb 28. Biometals. 2024. PMID: 38416244 Free PMC article. Review.
-
Parallel Evolution of Ameloblastic scpp Genes in Bony and Cartilaginous Vertebrates.Mol Biol Evol. 2022 May 3;39(5):msac099. doi: 10.1093/molbev/msac099. Mol Biol Evol. 2022. PMID: 35535508 Free PMC article.
-
Experimental recombining of repetitive motifs leads to large functional metallothioneins and demonstrates their modular evolvability potential.Protein Sci. 2025 Jan;34(1):e5247. doi: 10.1002/pro.5247. Protein Sci. 2025. PMID: 39673460 Free PMC article.
-
Modular Evolution and Population Variability of Oikopleura dioica Metallothioneins.Front Cell Dev Biol. 2021 Jul 2;9:702688. doi: 10.3389/fcell.2021.702688. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34277643 Free PMC article.
-
Evolution of Cd2+ and Cu+ binding in Helix pomatia metallothioneins.Metallomics. 2023 Oct 4;15(10):mfad057. doi: 10.1093/mtomcs/mfad057. Metallomics. 2023. PMID: 37738453 Free PMC article.
References
-
- Adamo GM, Lotti M, Tamas MJ, Brocca S.. 2012. Amplification of the CUP1 gene is associated with evolution of copper tolerance in Saccharomyces cerevisiae. Microbiology 158(Pt 9):2325–2335. - PubMed
-
- Artells E, Palacios O, Capdevila M, Atrian S.. 2013. Mammalian MT1 and MT2 metallothioneins differ in their metal binding abilities. Metallomics 5(10):1397–1410. - PubMed
-
- Baumann C, Beil A, Jurt S, Niederwanger M, Palacios O, Capdevila M, Atrian S, Dallinger R, Zerbe O.. 2017. Structural adaptation of a protein to increased metal stress: NMR structure of a marine snail metallothionein with an additional domain. Angew Chem Int Ed Engl. 56(16):4617–4622. - PubMed
-
- Beil A, Jurt S, Walser R, Schonhut T, Guntert P, Palacios O, Atrian S, Capdevila M, Dallinger R, Zerbe O.. 2019. The solution structure and dynamics of Cd-metallothionein from helix pomatia reveal optimization for binding Cd over Zn. Biochemistry 58(45):4570–4581. - PubMed
-
- Blindauer CA.2014. Metallothioneins. In: Maret W, Wedd A, editors. Binding, transport and storage of metal ions in biological cells. Cambridge: The Royal Society of Chemistry. p. 594–653.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

