External validation of the IMPROVE-DD risk assessment model for venous thromboembolism among inpatients with COVID-19
- PMID: 34146235
- PMCID: PMC8214061
- DOI: 10.1007/s11239-021-02504-5
External validation of the IMPROVE-DD risk assessment model for venous thromboembolism among inpatients with COVID-19
Abstract
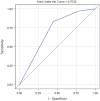
There is a need to discriminate which COVID-19 inpatients are at higher risk for venous thromboembolism (VTE) to inform prophylaxis strategies. The IMPROVE-DD VTE risk assessment model (RAM) has previously demonstrated good discrimination in non-COVID populations. We aimed to externally validate the IMPROVE-DD VTE RAM in medical patients hospitalized with COVID-19. This retrospective cohort study evaluated the IMPROVE-DD VTE RAM in adult patients with COVID-19 admitted to one of thirteen Northwell Health hospitals in the New York metropolitan area between March 1, 2020 and April 27, 2020. VTE was defined as new-onset symptomatic deep venous thrombosis or pulmonary embolism. To assess the predictive value of the RAM, the receiver operating characteristic (ROC) curve was plotted and the area under the curve (AUC) was calculated. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. Of 9407 patients who met study criteria, 274 patients developed VTE with a prevalence of 2.91%. The VTE rate was 0.41% for IMPROVE-DD score 0-1 (low risk), 1.21% for score 2-3 (moderate risk), and 5.30% for score ≥ 4 (high risk). Approximately 45.7% of patients were classified as high VTE risk, 33.3% moderate risk, and 21.0% low risk. Discrimination of low versus moderate-high VTE risk demonstrated sensitivity 0.971, specificity 0.215, PPV 0.036, and NPV 0.996. ROC AUC was 0.703. In this external validation study, the IMPROVE-DD VTE RAM demonstrated very good discrimination to identify hospitalized COVID-19 patients at low, moderate, and high VTE risk.
Keywords: COVID-19; Deep venous thrombosis; IMPROVE-DD; Pulmonary embolism; ROC curve; Risk assessment; Venous thromboembolism.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
ACS is a consultant for Boehringer Ingelheim, Janssen, Bayer, Bristol Myers Squibb, and Portola. No other authors have stated conflicts of interest.
Figures
References
-
- Al-Samkari H, Gupta S, Leaf RK, Wang W, Rosovsky RP, Brenner SK, Hayek SS, Berlin H, Kapoor R, Shaefi S, Melamed ML, Sutherland A, Radbel J, Green A, Garibaldi BT, Srivastava A, Leonberg-Yoo A, Shehata AM, Flythe JE, Rashidi A, Goyal N, Chan L, Mathews KS, Hedayati SS, Dy R, Toth-Manikowski SM, Zhang J, Mallappallil M, Redfern RE, Bansal AD, Short SAP, Vangel MG, Admon AJ, Semler MW, Bauer KA, Hernan MA, Leaf DE, S-C Investigators Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with COVID-19. Ann Intern Med. 2021 doi: 10.7326/M20-6739. - DOI - PMC - PubMed
-
- Cuker A, Tseng EK, Nieuwlaat R, Angchaisuksiri P, Blair C, Dane K, Davila J, DeSancho MT, Diuguid D, Griffin DO, Kahn SR, Klok FA, Lee AI, Neumann I, Pai A, Pai M, Righini M, Sanfilippo KM, Siegal D, Skara M, Touri K, Akl EA, Bou Akl I, Boulos M, Brignardello-Petersen R, Charide R, Chan M, Dearness K, Darzi AJ, Kolb P, Colunga-Lozano LE, Mansour R, Morgano GP, Morsi RZ, Noori A, Piggott T, Qiu Y, Roldan Y, Schünemann F, Stevens A, Solo K, Ventresca M, Wiercioch W, Mustafa RA, Schünemann HJ (2021) American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 5(3):872–888. 10.1182/bloodadvances.2020003763 - PMC - PubMed
-
- Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, Levi M, Samama CM, Thachil J, Giannis D, Douketis JD, Subcommittee on Perioperative CCTHotSSCotISoT, Haemostasis Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous