Chemoselective γ-Oxidation of β,γ-Unsaturated Amides with TEMPO
- PMID: 34146371
- PMCID: PMC8456850
- DOI: 10.1002/anie.202104023
Chemoselective γ-Oxidation of β,γ-Unsaturated Amides with TEMPO
Abstract
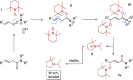
A chemoselective and robust protocol for the γ-oxidation of β,γ-unsaturated amides is reported. In this method, electrophilic amide activation, in a rare application to unsaturated amides, enables a regioselective reaction with TEMPO resulting in the title products. Radical cyclisation reactions and oxidation of the synthesised products highlight the synthetic utility of the products obtained.
Keywords: amides; chemoselectivity; oxidation; radical reactions; regioselectivity.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kaiser D., Bauer A., Lemmerer M., Maulide N., Chem. Soc. Rev. 2018, 47, 7899–7925. - PubMed
-
- None
-
- Wallach O., Justus Liebigs Ann. Chem. 1877, 184, 1–127;
-
- Baum J. S., Viehe H. G., J. Org. Chem. 1976, 41, 183–187.
-
- Falmagne J.-B., Escudero J., Taleb-Sahraoui S., Ghosez L., Angew. Chem. Int. Ed. Engl. 1981, 20, 879–880;
- Angew. Chem. 1981, 93, 926–931.
Grants and funding
LinkOut - more resources
Full Text Sources

