Niches that regulate stem cells and hematopoiesis in adult bone marrow
- PMID: 34146467
- PMCID: PMC8282762
- DOI: 10.1016/j.devcel.2021.05.018
Niches that regulate stem cells and hematopoiesis in adult bone marrow
Abstract
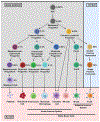
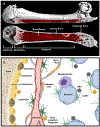
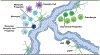
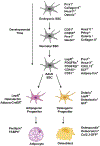
In mammals, hematopoietic stem cells (HSCs) engage in hematopoiesis throughout adult life within the bone marrow, where they produce the mature cells necessary to maintain blood cell counts and immune function. In the bone marrow and spleen, HSCs are sustained in perivascular niches (microenvironments) associated with sinusoidal blood vessels-specialized veins found only in hematopoietic tissues. Endothelial cells and perivascular leptin receptor+ stromal cells produce the known factors required to maintain HSCs and many restricted progenitors in the bone marrow. Various other cells synthesize factors that maintain other restricted progenitors or modulate HSC or niche function. Recent studies identified new markers that resolve some of the heterogeneity among stromal cells and refine the localization of restricted progenitor niches. Other recent studies identified ways in which niches regulate HSC function and hematopoiesis beyond growth factors. We summarize the current understanding of hematopoietic niches, review recent progress, and identify important unresolved questions.
Keywords: bone marrow; endothelial cell; hematopoietic stem cell; niche; restricted hematopoietic progenitor.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
References
-
- Baccin C, Al-Sabah J, Velten L, Helbling PM, Grunschlager F, Hernandez-Malmierca P, Nombela-Arrieta C, Steinmetz LM, Trumpp A, and Haas S (2020). Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat Cell Biol 22, 38–48. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

