Morphological characterization of Etv2 vascular explants using fractal analysis and atomic force microscopy
- PMID: 34146583
- PMCID: PMC8446305
- DOI: 10.1016/j.mvr.2021.104205
Morphological characterization of Etv2 vascular explants using fractal analysis and atomic force microscopy
Abstract
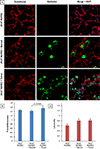
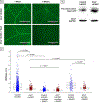
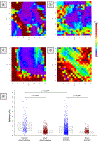
The rapid engraftment of vascular networks is critical for functional incorporation of tissue explants. However, existing methods for inducing angiogenesis utilize approaches that yield vasculature with poor temporal stability or inadequate mechanical integrity, which reduce their robustness in vivo. The transcription factor Ets variant 2 (Etv2) specifies embryonic hematopoietic and vascular endothelial cell (EC) development, and is transiently reactivated during postnatal vascular regeneration and tumor angiogenesis. This study investigates the role for Etv2 upregulation in forming stable vascular beds both in vitro and in vivo. Control and Etv2+ prototypical fetal-derived human umbilical vein ECs (HUVECs) and adult ECs were angiogenically grown into vascular beds. These vessel beds were characterized using fractal dimension and lacunarity, to quantify their branching complexity and space-filling homogeneity, respectively. Atomic force microscopy (AFM) was used to explore whether greater complexity and homogeneity lead to more mechanically stable vessels. Additionally, markers of EC integrity were used to probe for mechanistic clues. Etv2+ HUVECs exhibit greater branching, vessel density, and structural homogeneity, and decreased stiffness in vitro and in vivo, indicating a greater propensity for stable vessel formation. When co-cultured with colon tumor organoid tissue, Etv2+ HUVECs had decreased fractal dimension and lacunarity compared to Etv2+ HUVECs cultured alone, indicating that vessel density and homogeneity of vessel spacing increased due to the presence of Etv2. This study sets forth the novel concept that fractal dimension, lacunarity, and AFM are as informative as conventional angiogenic measurements, including vessel branching and density, to assess vascular perfusion and stability.
Keywords: Adipose-derived endothelial cells; Angiogenesis; Atomic force microscopy; Ets variant 2; F-actin; Fractal dimension; HUVECs; Lacunarity; Vascular engraftment.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures




References
-
- Cárdenas-Pérez, et al., 2018. Recent advances in atomic force microscopy for assessing the nanomechanical properties of food materials. Trends Food Sci Technol. 87, 59–72.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

