MS-Based HLA-II Peptidomics Combined With Multiomics Will Aid the Development of Future Immunotherapies
- PMID: 34146720
- PMCID: PMC8327157
- DOI: 10.1016/j.mcpro.2021.100116
MS-Based HLA-II Peptidomics Combined With Multiomics Will Aid the Development of Future Immunotherapies
Abstract
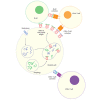
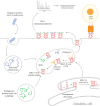
Immunotherapies have emerged to treat diseases by selectively modulating a patient's immune response. Although the roles of T and B cells in adaptive immunity have been well studied, it remains difficult to select targets for immunotherapeutic strategies. Because human leukocyte antigen class II (HLA-II) peptides activate CD4+ T cells and regulate B cell activation, proliferation, and differentiation, these peptide antigens represent a class of potential immunotherapy targets and biomarkers. To better understand the molecular basis of how HLA-II antigen presentation is involved in disease progression and treatment, systematic HLA-II peptidomics combined with multiomic analyses of diverse cell types in healthy and diseased states is required. For this reason, MS-based innovations that facilitate investigations into the interplay between disease pathologies and the presentation of HLA-II peptides to CD4+ T cells will aid in the development of patient-focused immunotherapies.
Keywords: CD4 T cells; HLA-II; antigen; immunopeptidome; neoantigen.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest S. A. C. is a member of the scientific advisory boards of Kymera, PTM Biolabs, and Seer and a scientific advisor to Pfizer and Biogen. J. G. A. is a past employee and shareholder of Neon Therapeutics, Inc (now BioNTech US). S. K., S. S., K. R. C., S. A. C., and J. G. A. are named co-inventors on patent application(s) related to HLA peptide motif technology that is related to topics in this article filed by The Broad Institute.
Figures

References
-
- Tran E., Turcotte S., Gros A., Robbins P.F., Lu Y.C., Dudley M.E., Wunderlich J.R., Somerville R.P., Hogan K., Hinrichs C.S., Parkhurst M.R., Yang J.C., Rosenberg S.A. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. - PMC - PubMed
-
- Kreiter S., Vormehr M., van de Roemer N., Diken M., Löwer M., Diekmann J., Boegel S., Schrörs B., Vascotto F., Castle J.C., Tadmor A.D., Schoenberger S.P., Huber C., Türeci Ö., Sahin U. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. - PMC - PubMed
-
- Quezada S.A., Simpson T.R., Peggs K.S., Merghoub T., Vider J., Fan X., Blasberg R., Yagita H., Muranski P., Antony P.A., Restifo N.P., Allison J.P. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010;207:637–650. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

