SARS-CoV2 infection impairs the metabolism and redox function of cellular glutathione
- PMID: 34146958
- PMCID: PMC8190457
- DOI: 10.1016/j.redox.2021.102041
SARS-CoV2 infection impairs the metabolism and redox function of cellular glutathione
Abstract
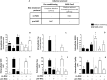
Viral infections sustain their replication cycle promoting a pro-oxidant environment in the host cell. In this context, specific alterations of the levels and homeostatic function of the tripeptide glutathione have been reported to play a causal role in the pro-oxidant and cytopathic effects (CPE) of the virus. In this study, these aspects were investigated for the first time in SARS-CoV2-infected Vero E6 cells, a reliable and well-characterized in vitro model of this infection. SARS-CoV2 markedly decreased the levels of cellular thiols, essentially lowering the reduced form of glutathione (GSH). Such an important defect occurred early in the CPE process (in the first 24 hpi). Thiol analysis in N-acetyl-Cys (NAC)-treated cells and membrane transporter expression data demonstrated that both a lowered uptake of the GSH biosynthesis precursor Cys and an increased efflux of cellular thiols, could play a role in this context. Increased levels of oxidized glutathione (GSSG) and protein glutathionylation were also observed along with upregulation of the ER stress marker PERK. The antiviral drugs Remdesivir (Rem) and Nelfinavir (Nel) influenced these changes at different levels, essentially confirming the importance or blocking viral replication to prevent GSH depletion in the host cell. Accordingly, Nel, the most potent antiviral in our in vitro study, produced a timely activation of Nrf2 transcription factor and a GSH enhancing response that synergized with NAC to restore GSH levels in the infected cells. Despite poor in vitro antiviral potency and GSH enhancing function, Rem treatment was found to prevent the SARS-CoV2-induced glutathionylation of cellular proteins. In conclusion, SARS-CoV2 infection impairs the metabolism of cellular glutathione. NAC and the antiviral Nel can prevent such defect in vitro.
Keywords: COVID-19; Glutathione; Nrf2; Protein glutathionylation; SARS-CoV-2; Thiols.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no competing financial or non-financial interests.
Figures




References
-
- Davies N.G., Klepac P., Liu Y., Prem K., Jit M., Eggo R.M., group C.C.-w. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020;26(8):1205–1211. - PubMed
-
- Eymieux S., Rouillé Y., Terrier O., Seron K., Blanchard E., Rosa-Calatrava M., Dubuisson J., Belouzard S., Roingeard P. Ultrastructural modifications induced by SARS-CoV-2 in Vero cells: a kinetic analysis of viral factory formation, viral particle morphogenesis and virion release. Cell. Mol. Life Sci. 2021;78(7):3565–3576. - PMC - PubMed
-
- Ogando N.S., Dalebout T.J., Zevenhoven-Dobbe J.C., Limpens R.W.A.L., van der Meer Y., Caly L., Druce J., de Vries J.J.C., Kikkert M., Bárcena M., Sidorov I., Snijder E.J. SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol. 2020;101(9):925–940. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

