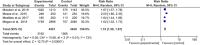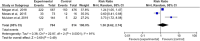Human papillomavirus self-sampling versus standard clinician-sampling for cervical cancer screening in sub-Saharan Africa: a systematic review and meta-analysis of randomized controlled trials
- PMID: 34147103
- PMCID: PMC8214270
- DOI: 10.1186/s13027-021-00380-5
Human papillomavirus self-sampling versus standard clinician-sampling for cervical cancer screening in sub-Saharan Africa: a systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Human papillomavirus (HPV) infection remains a major health threat in sub-Saharan Africa (SSA). HPV self-sampling could help find and treat cervical cancer at an early stage. We aimed to evaluate the effectiveness of HPV self-sampling over the standard health facility-based clinician-sampling for cervical cancer screening through a systematic review and meta-analysis of available randomized controlled trials.
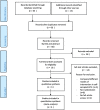
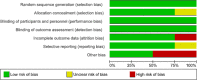
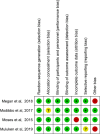
Method: We searched PubMed, Cochrane Central Register of Controlled Trials, ClinicalTrial.gov, and the WHO Global Health Library for articles in SSA published as of 31 May 2020. We followed the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 guidelines for the design and reporting of the results. We included randomized control trials that compared HPV self-sampling with the standard of care. The primary endpoint was uptake of cervical cancer screening service. The secondary endpoints were linkage to care, acceptability, screening frequency, and adverse events. We used RevMan V.5.3 software for statistical analysis. We computed random-effect model to provide pooled estimates of available data and I-squared (I2) test to assess heterogeneity.
Result: Of 77 citations, we included four trials from Nigeria, Ethiopia, Kenya, and Uganda, encompassing 8200 participants with age ranging from 25 to 65 years. The pooled analysis showed significantly higher uptake of cervical cancer screening in women who used HPV self-sampling (risk ratio [RR] 1.72, 95% CI 1.58-1.87; p = 0.01), while this had a considerable heterogeneity as explained by subgroup analysis. Uptake was higher in women who were offered sampling kit at home or work (RR 2.05, 95% CI 1.80-2.33) and those who's kit was mailed to or invited to a nearby health center (RR 1.65, 95% CI 1.58-1.72, I2 = 0%) than those screened with the standard of care. There was no difference between the two groups in the rate of linkage to care of positive cases (RR 1.30, 95% CI 0.90-2.74, I2 = 91%). HPV self-sampling was acceptable and easy to use. None of the trials compared the frequency of screening or adverse events.
Conclusion: HPV self-sampling is an effective and feasible alternative to the standard health facility-based clinician-sampling for cervical cancer screening in SSA. It could improve the uptake of cervical cancer screening and harness the global strategy towards elimination of cervical cancer by 2030.
Keywords: Cervical cancer screening; Human papillomavirus (HPV); Randomized controlled trial; Self-sampling; Sub-Saharan Africa; Systematic review and meta-analysis.
Conflict of interest statement
The authors declared that there are no conflicts of interest.
Figures
References
-
- https://www.google.com/search?q=globocan+2018+cervical+cancer. Retrieved May 21, 2020.
-
- Cervical cancer. https://www.who.int/westernpacific/health-topics/cervical-cancer. Retrieved May 21, 2020.
-
- Mboumba Bouassa RS, Prazuck T, Lethu T, Jenabian MA, Meye JF, Bélec L. Cervical cancer in sub-Saharan Africa: a preventable noncommunicable disease. https://pubmed.ncbi.nlm.nih.gov/28440679/. Retrieved May 21, 2020. - PubMed
-
- Projections of mortality and causes of death, 2016 to 2060. 2018. https://www.who.int/healthinfo/global_burden_disease/projections/en/. Retrieved May 21, 2020.
-
- WHO technical guidance and specifications of medical devices for screening and treatment of precancerous lesions in the prevention of cervical cancer. 2020. https://www.who.int/medical_devices/publications/tech_specs_precancerous.... Retrieved May 21, 2020.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials