Mass drug administration for the acceleration of malaria elimination in a region of Myanmar with artemisinin-resistant falciparum malaria: a cluster-randomised trial
- PMID: 34147154
- PMCID: PMC7614510
- DOI: 10.1016/S1473-3099(20)30997-X
Mass drug administration for the acceleration of malaria elimination in a region of Myanmar with artemisinin-resistant falciparum malaria: a cluster-randomised trial
Abstract
Background: To contain multidrug-resistant Plasmodium falciparum, malaria elimination in the Greater Mekong subregion needs to be accelerated while current antimalarials remain effective. We evaluated the safety, effectiveness, and potential resistance selection of dihydroartemisinin-piperaquine mass drug administration (MDA) in a region with artemisinin resistance in Myanmar.
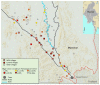
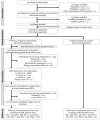
Methods: We did a cluster-randomised controlled trial in rural community clusters in Kayin (Karen) state in southeast Myanmar. Malaria prevalence was assessed using ultrasensitive quantitative PCR (uPCR) in villages that were operationally suitable for MDA (villages with community willingness, no other malaria control campaigns, and a population of 50-1200). Villages were eligible to participate if the prevalence of malaria (all species) in adults was greater than 30% or P falciparum prevalence was greater than 10% (or both). Contiguous villages were combined into clusters. Eligible clusters were paired based on P falciparum prevalence (estimates within 10%) and proximity. Community health workers provided routine malaria case management and distributed long-lasting insecticidal bed-nets (LLINs) in all clusters. Randomisation of clusters (1:1) to the MDA intervention group or control group was by public coin-flip. Group allocations were not concealed. Three MDA rounds (3 days of supervised dihydroartemisinin-piperaquine [target total dose 7 mg/kg dihydroartemisinin and 55 mg/kg piperaquine] and single low-dose primaquine [target dose 0·25 mg base per kg]) were delivered to intervention clusters. Parasitaemia prevalence was assessed at 3, 5, 10, 15, 21, 27, and 33 months. The primary outcomes were P falciparum prevalence at months 3 and 10. All clusters were included in the primary analysis. Adverse events were monitored from the first MDA dose until 1 month after the final dose, or until resolution of any adverse event occurring during follow-up. This trial is registered with ClinicalTrials.gov, NCT01872702.
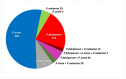
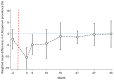
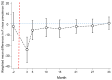
Findings: Baseline uPCR malaria surveys were done in January, 2015, in 43 villages that were operationally suitable for MDA (2671 individuals). 18 villages met the eligibility criteria. Three villages in close proximity were combined into one cluster because a border between them could not be defined. This gave a total of 16 clusters in eight pairs. In the intervention clusters, MDA was delivered from March 4 to March 17, from March 30 to April 10, and from April 27 to May 10, 2015. The weighted mean absolute difference in P falciparum prevalence in the MDA group relative to the control group was -10·6% (95% CI -15·1 to -6·1; p=0·0008) at month 3 and -4·5% (-10·9 to 1·9; p=0·14) at month 10. At month 3, the weighted P falciparum prevalence was 1·4% (0·6 to 3·6; 12 of 747) in the MDA group and 10·6% (7·0 to 15·6; 56 of 485) in the control group. Corresponding prevalences at month 10 were 3·2% (1·5 to 6·8; 34 of 1013) and 5·8% (2·5 to 12·9; 33 of 515). Adverse events were reported for 151 (3·6%) of 4173 treated individuals. The most common adverse events were dizziness (n=109) and rash or itching (n=20). No treatment-related deaths occurred.
Interpretation: In this low-transmission setting, the substantial reduction in P falciparum prevalence resulting from support of community case management was accelerated by MDA. In addition to supporting community health worker case management and LLIN distribution, malaria elimination programmes should consider using MDA to reduce P falciparum prevalence rapidly in foci of higher transmission.
Funding: The Global Fund to Fight AIDS, Tuberculosis and Malaria.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures






Comment in
-
Bold measures to accelerate malaria elimination.Lancet Infect Dis. 2021 Nov;21(11):1480-1481. doi: 10.1016/S1473-3099(21)00003-7. Epub 2021 Jun 18. Lancet Infect Dis. 2021. PMID: 34147153 No abstract available.
References
-
- WHO. World Malaria Report 2016. 2016
-
- Saunders DL, Vanachayangkul P, Lon C. Dihydroartemisinin-piperaquine failure in Cambodia. The New England journal of medicine. 2014;371(5):484–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

