Long-term efficacy and safety of CT-P6 versus trastuzumab in patients with HER2-positive early breast cancer: final results from a randomized phase III trial
- PMID: 34148205
- PMCID: PMC8272708
- DOI: 10.1007/s10549-021-06240-5
Long-term efficacy and safety of CT-P6 versus trastuzumab in patients with HER2-positive early breast cancer: final results from a randomized phase III trial
Abstract
Purpose: Equivalent efficacy was demonstrated for the biosimilar CT-P6 and trastuzumab following neoadjuvant therapy for patients with human epidermal growth factor receptor-2 (HER2)-positive early breast cancer. Following adjuvant treatment, efficacy and safety were comparable between treatments. We report updated safety and efficacy data after up to 3 years' follow-up.
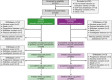
Methods: Following neoadjuvant chemotherapy with CT-P6/trastuzumab, patients underwent surgery and continued receiving adjuvant CT-P6/trastuzumab. The primary endpoint (previously reported) was pathological complete response. Time-to-event analyses (disease-free survival [DFS], progression-free survival [PFS], and overall survival [OS]), study drug-related and cardiac adverse events, and immunogenicity were assessed during post-treatment follow-up.
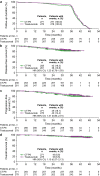
Results: Most patients entered the follow-up period (CT-P6: 259 [95.6%]; trastuzumab: 269 [96.8%]). After a median follow-up of 38.7 (CT-P6) and 39.6 (trastuzumab) months, medians were not reached for time-to-event parameters; estimated hazard ratios (HRs) and 3-year survival rates were similar between groups. Estimated HRs (95% confidence intervals) for CT-P6 versus trastuzumab were 1.23 (0.78-1.93) for DFS, 1.31 (0.86-2.01) for PFS, and 1.10 (0.57-2.13) for OS (intention-to-treat population). Safety findings were comparable between groups for the overall study and follow-up period, including study drug-related cardiac disorders (CT-P6: 22 [8.1%] patients; trastuzumab: 24 [8.6%] patients [overall]) and decreases in left ventricular ejection fraction. Immunogenicity was similar between groups.
Conclusion: The similarity of the time-to-event analyses between CT-P6 and trastuzumab supports the equivalence in terms of efficacy established for the primary endpoint. CT-P6 was well tolerated, with comparable safety and immunogenicity to trastuzumab. ClinicalTrials.gov: NCT02162667 (registered June 13, 2014).
Keywords: Adjuvant therapy; Biosimilar; CT-P6; HER2-positive early breast cancer; Neoadjuvant therapy; Trastuzumab.
© 2021. The Author(s).
Conflict of interest statement
JS has served on scientific advisory boards for Alveo Technologies, Inc., BenevolentAI, Celltrion, Certis Oncology Solutions, Inc., Eli Lilly and Company, Greenmantle, Heat Biologics, Inc., Replete Pharmaceuticals Private Ltd, Vaccitech Ltd, and Zedsen Ltd; has consulted for Lansdowne Partners Ltd and Vitruvian Partners; sits on the Board of Directors for BB Biotech Healthcare Trust PLC; and is Editor-in-Chief of Oncogene, outside the submitted work. VM reports grants from Pfizer Inc. and Samsung Bioepis Co., Ltd, outside the submitted work. AEE reports grants from Celltrion, Inc. during the conduct of the study, and grants from AstraZeneca PLC, Pfizer Inc., and F. Hoffmann-La Roche AG outside the submitted work. SJL is employed by and has stock options with Celltrion, Inc. SK is employed by Celltrion, Inc. YVB, VB, AM, GD, EZ, DB, JP, RKL and BT have no competing interests to declare.
Figures
References
-
- National Comprehensive Cancer Center (2021) Breast cancer. Version 2.2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 24 March 2021
-
- US Food and Drug Administration (2019) Herzuma (trastuzumab-pkrb) highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761091s001s002.... Accessed 24 March 2021
-
- US Food and Drug Administration (2018) Herceptin (trastuzumab) highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103792s5347lbl.... Accessed 24 March 2021
-
- European Medicines Agency (2020) Herceptin summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/herceptin-epa.... Accessed 24 March 2021
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

