Epigenetic Impacts of Early Life Stress in Fetal Alcohol Spectrum Disorders Shape the Neurodevelopmental Continuum
- PMID: 34149355
- PMCID: PMC8209299
- DOI: 10.3389/fnmol.2021.671891
Epigenetic Impacts of Early Life Stress in Fetal Alcohol Spectrum Disorders Shape the Neurodevelopmental Continuum
Abstract
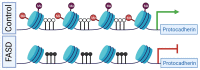
Neurodevelopment in humans is a long, elaborate, and highly coordinated process involving three trimesters of prenatal development followed by decades of postnatal development and maturation. Throughout this period, the brain is highly sensitive and responsive to the external environment, which may provide a range of inputs leading to positive or negative outcomes. Fetal alcohol spectrum disorders (FASD) result from prenatal alcohol exposure (PAE). Although the molecular mechanisms of FASD are not fully characterized, they involve alterations to the regulation of gene expression via epigenetic marks. As in the prenatal stages, the postnatal period of neurodevelopment is also sensitive to environmental inputs. Often this sensitivity is reflected in children facing adverse conditions, such as maternal separation. This exposure to early life stress (ELS) is implicated in the manifestation of various behavioral abnormalities. Most FASD research has focused exclusively on the effect of prenatal ethanol exposure in isolation. Here, we review the research into the effect of prenatal ethanol exposure and ELS, with a focus on the continuum of epigenomic and transcriptomic alterations. Interestingly, a select few experiments have assessed the cumulative effect of prenatal alcohol and postnatal maternal separation stress. Regulatory regions of different sets of genes are affected by both treatments independently, and a unique set of genes are affected by the combination of treatments. Notably, epigenetic and gene expression changes converge at the clustered protocadherin locus and oxidative stress pathway. Functional studies using epigenetic editing may elucidate individual contributions of regulatory regions for hub genes and further profiling efforts may lead to the development of non-invasive methods to identify children at risk. Taken together, the results favor the potential to improve neurodevelopmental outcomes by epigenetic management of children born with FASD using favorable postnatal conditions with or without therapeutic interventions.
Keywords: DNA methylation; clustered protocadherins (Pcdhs); early life stress (ELS); epigenetics; fetal alcohol spectrum disorders (FASD); neurodevelopment; oxidative stress; prenatal alcohol exposure (PAE).
Copyright 2021 Alberry, Laufer, Chater-Diehl and Singh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

