Multimodal Optical Imaging to Investigate Spatiotemporal Changes in Cerebrovascular Function in AUDA Treatment of Acute Ischemic Stroke
- PMID: 34149359
- PMCID: PMC8209306
- DOI: 10.3389/fncel.2021.655305
Multimodal Optical Imaging to Investigate Spatiotemporal Changes in Cerebrovascular Function in AUDA Treatment of Acute Ischemic Stroke
Abstract
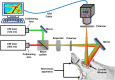
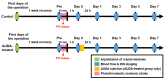
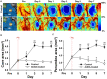
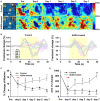
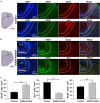
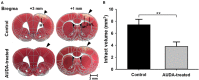
Administration of 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) has been demonstrated to alleviate infarction following ischemic stroke. Reportedly, the main effect of AUDA is exerting anti-inflammation and neovascularization via the inhibition of soluble epoxide hydrolase. However, the major contribution of this anti-inflammation and neovascularization effect in the acute phase of stroke is not completely elucidated. To investigate the neuroprotective effects of AUDA in acute ischemic stroke, we combined laser speckle contrast imaging and optical intrinsic signal imaging techniques with the implantation of a lab-designed cranial window. Forepaw stimulation was applied to assess the functional changes via measuring cerebral metabolic rate of oxygen (CMRO2) that accompany neural activity. The rats that received AUDA in the acute phase of photothrombotic ischemia stroke showed a 30.5 ± 8.1% reduction in the ischemic core, 42.3 ± 15.1% reduction in the ischemic penumbra (p < 0.05), and 42.1 ± 4.6% increase of CMRO2 in response to forepaw stimulation at post-stroke day 1 (p < 0.05) compared with the control group (N = 10 for each group). Moreover, at post-stroke day 3, increased functional vascular density was observed in AUDA-treated rats (35.9 ± 1.9% higher than that in the control group, p < 0.05). At post-stroke day 7, a 105.4% ± 16.4% increase of astrocytes (p < 0.01), 30.0 ± 10.9% increase of neurons (p < 0.01), and 65.5 ± 15.0% decrease of microglia (p < 0.01) were observed in the penumbra region in AUDA-treated rats (N = 5 for each group). These results suggested that AUDA affects the anti-inflammation at the beginning of ischemic injury and restores neuronal metabolic rate of O2 and tissue viability. The neovascularization triggered by AUDA restored CBF and may contribute to ischemic infarction reduction at post-stroke day 3. Moreover, for long-term neuroprotection, astrocytes in the penumbra region may play an important role in protecting neurons from apoptotic injury.
Keywords: AUDA; cranial window; ischemic stroke; multimodal optical imaging system; neovascularization; penumbra.
Copyright © 2021 Wang, Chen, Yang, Lo, Pan, Liang, Wang, Yang, Kuo, Lin, Chou, Lin and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Soluble epoxide hydrolase inhibition enhances anti-inflammatory and antioxidative processes, modulates microglia polarization, and promotes recovery after ischemic stroke.Neuropsychiatr Dis Treat. 2019 Oct 15;15:2927-2941. doi: 10.2147/NDT.S210403. eCollection 2019. Neuropsychiatr Dis Treat. 2019. PMID: 31686827 Free PMC article.
-
An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats.J Cardiovasc Pharmacol. 2005 Dec;46(6):842-8. doi: 10.1097/01.fjc.0000189600.74157.6d. J Cardiovasc Pharmacol. 2005. PMID: 16306811 Free PMC article.
-
Genetic deletion or pharmacological inhibition of soluble epoxide hydrolase reduces brain damage and attenuates neuroinflammation after intracerebral hemorrhage.J Neuroinflammation. 2017 Nov 25;14(1):230. doi: 10.1186/s12974-017-1005-4. J Neuroinflammation. 2017. PMID: 29178914 Free PMC article.
-
Neuroprotection targeting ischemic penumbra and beyond for the treatment of ischemic stroke.Neurol Res. 2012 May;34(4):331-7. doi: 10.1179/1743132812Y.0000000020. Neurol Res. 2012. PMID: 22643076 Review.
-
Extension of therapeutic window in ischemic stroke by selective mismatch imaging.Int J Stroke. 2019 Jun;14(4):351-358. doi: 10.1177/1747493019840936. Epub 2019 Apr 1. Int J Stroke. 2019. PMID: 30935350 Review.
Cited by
-
Cranial and Spinal Window Preparation for in vivo Optical Neuroimaging in Rodents and Related Experimental Techniques.Exp Neurobiol. 2022 Jun 30;31(3):131-146. doi: 10.5607/en22015. Exp Neurobiol. 2022. PMID: 35786637 Free PMC article. Review.
-
Acupuncture Alleviates Blood-Brain Barrier Damage After Delayed rtPA Thrombolysis for Acute Ischemic Stroke by Regulating Ferroptosis.Brain Behav. 2025 Jul;15(7):e70644. doi: 10.1002/brb3.70644. Brain Behav. 2025. PMID: 40637138 Free PMC article.
-
The Critical Role of Equilibrative Nucleoside Transporter-2 in Modulating Cerebral Damage and Vascular Dysfunction in Mice with Brain Ischemia-Reperfusion.Pharm Res. 2023 Nov;40(11):2541-2554. doi: 10.1007/s11095-023-03565-2. Epub 2023 Jul 27. Pharm Res. 2023. PMID: 37498500
References
-
- Autio J., Kawaguchi H., Saito S., Aoki I., Obata T., Masamoto K., et al. (2011). Spatial frequency-based analysis of mean red blood cell speed in single microvessels: investigation of microvascular perfusion in rat cerebral cortex. PLoS One 6:e24056. 10.1371/journal.pone.0024056 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

