Modeling the Glomerular Filtration Barrier and Intercellular Crosstalk
- PMID: 34149462
- PMCID: PMC8206562
- DOI: 10.3389/fphys.2021.689083
Modeling the Glomerular Filtration Barrier and Intercellular Crosstalk
Abstract
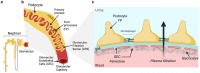
The glomerulus is a compact cluster of capillaries responsible for blood filtration and initiating urine production in the renal nephrons. A trilaminar structure in the capillary wall forms the glomerular filtration barrier (GFB), composed of glycocalyx-enriched and fenestrated endothelial cells adhering to the glomerular basement membrane and specialized visceral epithelial cells, podocytes, forming the outermost layer with a molecular slit diaphragm between their interdigitating foot processes. The unique dynamic and selective nature of blood filtration to produce urine requires the functionality of each of the GFB components, and hence, mimicking the glomerular filter in vitro has been challenging, though critical for various research applications and drug screening. Research efforts in the past few years have transformed our understanding of the structure and multifaceted roles of the cells and their intricate crosstalk in development and disease pathogenesis. In this review, we present a new wave of technologies that include glomerulus-on-a-chip, three-dimensional microfluidic models, and organoids all promising to improve our understanding of glomerular biology and to enable the development of GFB-targeted therapies. Here, we also outline the challenges and the opportunities of these emerging biomimetic systems that aim to recapitulate the complex glomerular filter, and the evolving perspectives on the sophisticated repertoire of cellular signaling that comprise the glomerular milieu.
Keywords: 3D model; crosstalk; glomerular endothelial cell; glomerular filtration barrier; in vitro; podocyte.
Copyright © 2021 Ebefors, Lassén, Anandakrishnan, Azeloglu and Daehn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

