Metformin Improves Autonomic Nervous System Imbalance and Metabolic Dysfunction in Monosodium L-Glutamate-Treated Rats
- PMID: 34149616
- PMCID: PMC8212417
- DOI: 10.3389/fendo.2021.660793
Metformin Improves Autonomic Nervous System Imbalance and Metabolic Dysfunction in Monosodium L-Glutamate-Treated Rats
Abstract
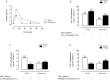
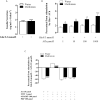
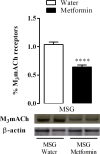
Metformin is an antidiabetic drug used for the treatment of diabetes and metabolic diseases. Imbalance in the autonomic nervous system (ANS) is associated with metabolic diseases. This study aimed to test whether metformin could improve ANS function in obese rats. Obesity was induced by neonatal treatment with monosodium L-glutamate (MSG). During 21-100 days of age, MSG-rats were treated with metformin 250 mg/kg body weight/day or saline solution. Rats were euthanized to evaluate biometric and biochemical parameters. ANS electrical activity was recorded and analyzed. Metformin normalized the hypervagal response in MSG-rats. Glucose-stimulated insulin secretion in isolated pancreatic islets increased in MSG-rats, while the cholinergic response decreased. Metformin treatment normalized the cholinergic response, which involved mostly the M3 muscarinic acetylcholine receptor (M3 mAChR) in pancreatic beta-cells. Protein expression of M3 mAChRs increased in MSG-obesity rats, while metformin treatment decreased the protein expression by 25%. In conclusion, chronic metformin treatment was effective in normalizing ANS activity and alleviating obesity in MSG-rats.
Keywords: MSG-obese rats; acetylcholine; autonomic nervous system; insulin secretion; metformin.
Copyright © 2021 Franco, Previate, Trombini, Miranda, Barella, Saavedra, de Oliveira, Prates, Tófolo, Ribeiro, Pavanello, Malta, Martins, Moreira, Matiusso, Francisco, Alves, de Moraes, de Sant Anna, de Castro Prado, Gomes, Vieira and de Freitas Mathias.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

