Sublingual Bacterial Vaccination Reduces Recurrent Infections in Patients With Autoimmune Diseases Under Immunosuppressant Treatment
- PMID: 34149711
- PMCID: PMC8212043
- DOI: 10.3389/fimmu.2021.675735
Sublingual Bacterial Vaccination Reduces Recurrent Infections in Patients With Autoimmune Diseases Under Immunosuppressant Treatment
Abstract
Introduction: Conventional or biologic disease-modifying anti-rheumatic drugs (DMARDs) are the mainstay of treatment for systemic autoimmune disease (SAD). Infectious complications are a major concern in their use.
Objective: To evaluate the clinical benefit of sublingual mucosal polybacterial vaccines (MV130 and MV140), used to prevent recurrent respiratory and urinary tract infections, in patients with SAD and secondary recurrent infections following conventional or biologic DMARDs.
Methods: An observational study in SAD patients with recurrent respiratory tract infections (RRTI) and/or recurrent urinary tract infections (RUTI) was carried out. All patients underwent mucosal (sublingual) vaccination with MV130 for RRTI or with MV140 for RUTI daily for 3 months. Clinical evaluation was assessed during 12 months of follow-up after the first dose, i.e., 3 months under treatment and 9 months once discontinued, and compared with the previous year.
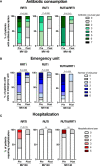
Results: Forty-one out of 55 patients completed 1-year follow-up. All patients were on either conventional or biologic DMARDs. A significant decrease in the frequency of RUTI (p<0.001), lower respiratory tract infections (LRTI) (p=0.009) and upper respiratory tract infections (URTI) (p=0.006) at 12-mo with respect to the previous year was observed. Antibiotic prescriptions and unscheduled medical visits decreased significantly (p<0.020) in all groups. Hospitalization rate also declined in patients with RRTI (p=0.019). The clinical benefit demonstrated was concomitant to a significant increase in both anti-S. pneumoniae IgA and IgG antibodies following MV130 vaccination.
Conclusions: Sublingual polybacterial vaccines prevent recurrent infections in patients with SAD under treatment with immunosuppressant therapies, supporting a broad non-specific anti-infectious effect in these patients.
Keywords: MV130; MV140; biological therapies; mucosal bacterial vaccines; recurrent infections; systemic autoimmune disease.
Copyright © 2021 Sánchez-Ramón, Fernández-Paredes, Saz-Leal, Diez-Rivero, Ochoa-Grullón, Morado, Macarrón, Martínez, Villaverde, de la Peña, Conejero, Hernández-Llano, Cordero, Fernández-Arquero, Gutierrez and Candelas.
Conflict of interest statement
PS-L, CD-R and LC are employees of Inmunotek S.L. SS-R has received in the past a fee as speaker from Inmunotek S.L. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Subesinghe S, Rutherford AI, Byng-Maddick R, Hyrich KL, Galloway JB. Biologic Prescribing Decisions Following Serious Infection: Results From the British Society for Rheumatology Biologics Register-Rheumatoid Arthritis. Rheumatol (Oxford) (2018) 57:2096–100. 10.1093/rheumatology/key198 - DOI - PubMed
-
- Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic Infections in Rheumatoid Arthritis Patients Exposed to Biologic Therapy: Results From the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatol (Oxford) (2018) 57:997–1001. 10.1093/rheumatology/key023 - DOI - PubMed
-
- Carmona L, Descalzo MA, Ruiz-Montesinos D, Manero-Ruiz FJ, Perez-Pampin E, Gomez-Reino JJ, et al. . Safety and Retention Rate of Off-Label Uses of TNF Antagonists in Rheumatic Conditions: Data From the Spanish Registry BIOBADASER 2.0. Rheumatol (Oxford) (2011) 50:85–92. 10.1093/rheumatology/keq207 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

