A Bittersweet Response to Infection in Diabetes; Targeting Neutrophils to Modify Inflammation and Improve Host Immunity
- PMID: 34149714
- PMCID: PMC8209466
- DOI: 10.3389/fimmu.2021.678771
A Bittersweet Response to Infection in Diabetes; Targeting Neutrophils to Modify Inflammation and Improve Host Immunity
Abstract
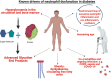
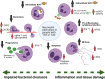
Chronic and recurrent infections occur commonly in both type 1 and type 2 diabetes (T1D, T2D) and increase patient morbidity and mortality. Neutrophils are professional phagocytes of the innate immune system that are critical in pathogen handling. Neutrophil responses to infection are dysregulated in diabetes, predominantly mediated by persistent hyperglycaemia; the chief biochemical abnormality in T1D and T2D. Therapeutically enhancing host immunity in diabetes to improve infection resolution is an expanding area of research. Individuals with diabetes are also at an increased risk of severe coronavirus disease 2019 (COVID-19), highlighting the need for re-invigorated and urgent focus on this field. The aim of this review is to explore the breadth of previous literature investigating neutrophil function in both T1D and T2D, in order to understand the complex neutrophil phenotype present in this disease and also to focus on the development of new therapies to improve aberrant neutrophil function in diabetes. Existing literature illustrates a dual neutrophil dysfunction in diabetes. Key pathogen handling mechanisms of neutrophil recruitment, chemotaxis, phagocytosis and intracellular reactive oxygen species (ROS) production are decreased in diabetes, weakening the immune response to infection. However, pro-inflammatory neutrophil pathways, mainly neutrophil extracellular trap (NET) formation, extracellular ROS generation and pro-inflammatory cytokine generation, are significantly upregulated, causing damage to the host and perpetuating inflammation. Reducing these proinflammatory outputs therapeutically is emerging as a credible strategy to improve infection resolution in diabetes, and also more recently COVID-19. Future research needs to drive forward the exploration of novel treatments to improve infection resolution in T1D and T2D to improve patient morbidity and mortality.
Keywords: COVID-19; NETosis; hyperglycaemia; infection; inflammation; neutrophil; type 1 diabetes; type 2 diabetes.
Copyright © 2021 Dowey, Iqbal, Heller, Sabroe and Prince.
Conflict of interest statement
SH undertakes consultancy for Eli Lilly, Sanofi Aventis, NovoNordisk, Zealand Pharma, and have been on speaker panels for NovoNordisk and Astra Zeneca. These companies’ products have effects on hypoglycaemia when treating individuals with diabetes and are therefore related to this paper. AI has consulted for OrbiMed LLC and received educational grant support from Sanofi S.A. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
NETosis and Neutrophil Extracellular Traps in COVID-19: Immunothrombosis and Beyond.Front Immunol. 2022 Mar 2;13:838011. doi: 10.3389/fimmu.2022.838011. eCollection 2022. Front Immunol. 2022. PMID: 35309344 Free PMC article. Review.
-
COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps.Mediators Inflamm. 2020 Dec 2;2020:8829674. doi: 10.1155/2020/8829674. eCollection 2020. Mediators Inflamm. 2020. PMID: 33343232 Free PMC article. Review.
-
E-cigarette use increases susceptibility to bacterial infection by impairment of human neutrophil chemotaxis, phagocytosis, and NET formation.Am J Physiol Cell Physiol. 2020 Jan 1;318(1):C205-C214. doi: 10.1152/ajpcell.00045.2019. Epub 2019 Oct 30. Am J Physiol Cell Physiol. 2020. PMID: 31664858 Free PMC article.
-
Dysregulated Neutrophil Phenotype and Function in Hospitalised Non-ICU COVID-19 Pneumonia.Cells. 2022 Sep 16;11(18):2901. doi: 10.3390/cells11182901. Cells. 2022. PMID: 36139476 Free PMC article.
-
With Friends Like These: The Complex Role of Neutrophils in the Progression of Severe Pneumonia.Front Cell Infect Microbiol. 2017 May 1;7:160. doi: 10.3389/fcimb.2017.00160. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28507954 Free PMC article. Review.
Cited by
-
Neutrophils in Mycobacterium tuberculosis.Vaccines (Basel). 2023 Mar 12;11(3):631. doi: 10.3390/vaccines11030631. Vaccines (Basel). 2023. PMID: 36992214 Free PMC article. Review.
-
Connection between Impaired Fasting Glucose or Type 2 Diabetes Mellitus and Sepsis: A 10-Year Observational Data from the National Health Screening Cohort.Diabetes Metab J. 2025 May;49(3):485-497. doi: 10.4093/dmj.2024.0387. Epub 2025 Feb 17. Diabetes Metab J. 2025. PMID: 39957312 Free PMC article.
-
Heparin-binding protein as a predictor of mortality in patients with diabetes mellitus and community-acquired pneumonia in intensive care unit : a propensity score matched study.World J Emerg Med. 2024;15(4):263-272. doi: 10.5847/wjem.j.1920-8642.2024.033. World J Emerg Med. 2024. PMID: 39050224 Free PMC article.
-
Immunomodulatory Bandage for Accelerated Healing of Diabetic Wounds.ACS Bio Med Chem Au. 2022 Aug 17;2(4):409-418. doi: 10.1021/acsbiomedchemau.1c00063. Epub 2022 Apr 4. ACS Bio Med Chem Au. 2022. PMID: 35996477 Free PMC article.
-
Do people with diabetes have a higher risk of developing postoperative endophthalmitis after cataract surgery? A systematic review and meta-analysis.J Ophthalmic Inflamm Infect. 2025 Mar 12;15(1):24. doi: 10.1186/s12348-025-00483-9. J Ophthalmic Inflamm Infect. 2025. PMID: 40072731 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

