Low Energy Electron Irradiation Is a Potent Alternative to Gamma Irradiation for the Inactivation of (CAR-)NK-92 Cells in ATMP Manufacturing
- PMID: 34149724
- PMCID: PMC8212864
- DOI: 10.3389/fimmu.2021.684052
Low Energy Electron Irradiation Is a Potent Alternative to Gamma Irradiation for the Inactivation of (CAR-)NK-92 Cells in ATMP Manufacturing
Abstract
Background: With increasing clinical use of NK-92 cells and their CAR-modified derivatives in cancer immunotherapy, there is a growing demand for efficient production processes of these "off-the-shelf" therapeutics. In order to ensure safety and prevent the occurrence of secondary tumors, (CAR-)NK-92 cell proliferation has to be inactivated before transfusion. This is commonly achieved by gamma irradiation. Recently, we showed proof of concept that low energy electron irradiation (LEEI) is a new method for NK-92 inactivation. LEEI has several advantages over gamma irradiation, including a faster reaction time, a more reproducible dose rate and much less requirements on radiation shielding. Here, LEEI was further evaluated as a promising alternative to gamma irradiation yielding cells with highly maintained cytotoxic effector function.
Methods: Effectiveness and efficiency of LEEI and gamma irradiation were analyzed using NK-92 and CD123-directed CAR-NK-92 cells. LEE-irradiated cells were extensively characterized and compared to gamma-irradiated cells via flow cytometry, cytotoxicity assays, and comet assays, amongst others.
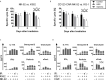
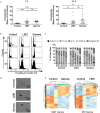
Results: Our results show that both irradiation methods caused a progressive decrease in cell viability and are, therefore, suitable for inhibition of cell proliferation. Notably, the NK-mediated specific lysis of tumor cells was maintained at stable levels for three days post-irradiation, with a trend towards higher activities after LEEI treatment as compared to gamma irradiation. Both gamma irradiation as well as LEEI led to substantial DNA damage and an accumulation of irradiated cells in the G2/M cell cycle phases. In addition, transcriptomic analysis of irradiated cells revealed approximately 12-fold more differentially expressed genes two hours after gamma irradiation, compared to LEEI. Analysis of surface molecules revealed an irradiation-induced decrease in surface expression of CD56, but no changes in the levels of the activating receptors NKp46, NKG2D, or NKp30.
Conclusions: The presented data show that LEEI inactivates (CAR-)NK-92 cells as efficiently as gamma irradiation, but with less impact on the overall gene expression. Due to logistic advantages, LEEI might provide a superior alternative for the manufacture of (CAR-)NK-92 cells for clinical application.
Keywords: CAR-NK-92; NK-92; acute myeloid leukemia; chimeric antigen receptor; gamma irradiation; immune cell therapy; low energy electron irradiation; off-the-shelf therapy.
Copyright © 2021 Walcher, Kistenmacher, Sommer, Böhlen, Ziemann, Dehmel, Braun, Tretbar, Klöß, Schambach, Morgan, Löffler, Kämpf, Blumert, Reiche, Beckmann, König, Standfest, Thoma, Makert, Ulbert, Kossatz-Böhlert, Köhl, Dünkel and Fricke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Optimization of Human NK Cell Manufacturing: Fully Automated Separation, Improved Ex Vivo Expansion Using IL-21 with Autologous Feeder Cells, and Generation of Anti-CD123-CAR-Expressing Effector Cells.Hum Gene Ther. 2017 Oct;28(10):897-913. doi: 10.1089/hum.2017.157. Epub 2017 Aug 15. Hum Gene Ther. 2017. PMID: 28810809
-
Irradiated chimeric antigen receptor engineered NK-92MI cells show effective cytotoxicity against CD19+ malignancy in a mouse model.Cytotherapy. 2020 Oct;22(10):552-562. doi: 10.1016/j.jcyt.2020.06.003. Epub 2020 Aug 1. Cytotherapy. 2020. PMID: 32747298
-
Development of Automated Separation, Expansion, and Quality Control Protocols for Clinical-Scale Manufacturing of Primary Human NK Cells and Alpharetroviral Chimeric Antigen Receptor Engineering.Hum Gene Ther Methods. 2019 Jun;30(3):102-120. doi: 10.1089/hgtb.2019.039. Epub 2019 May 16. Hum Gene Ther Methods. 2019. PMID: 30997855 Free PMC article.
-
Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy.Acta Pharmacol Sin. 2018 Feb;39(2):167-176. doi: 10.1038/aps.2017.125. Epub 2017 Sep 7. Acta Pharmacol Sin. 2018. PMID: 28880014 Free PMC article. Review.
-
Reformation in chimeric antigen receptor based cancer immunotherapy: Redirecting natural killer cell.Biochim Biophys Acta Rev Cancer. 2018 Apr;1869(2):200-215. doi: 10.1016/j.bbcan.2018.01.005. Epub 2018 Jan 31. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29378229 Review.
Cited by
-
iPSCs in NK Cell Manufacturing and NKEV Development.Front Immunol. 2022 Jul 8;13:890894. doi: 10.3389/fimmu.2022.890894. eCollection 2022. Front Immunol. 2022. PMID: 35874677 Free PMC article. Review.
-
A Novel Therapeutic Effect of a New Variant of CTLA4-Ig with Four Antennas That Are Terminally Capped with Sialic Acid in the CTLA4 Region.Biomol Ther (Seoul). 2022 Nov 1;30(6):529-539. doi: 10.4062/biomolther.2022.071. Epub 2022 Sep 29. Biomol Ther (Seoul). 2022. PMID: 36172704 Free PMC article.
-
Two for one: targeting BCMA and CD19 in B-cell malignancies with off-the-shelf dual-CAR NK-92 cells.J Transl Med. 2022 Mar 14;20(1):124. doi: 10.1186/s12967-022-03326-6. J Transl Med. 2022. PMID: 35287669 Free PMC article.
-
Engineered NK Cells Against Cancer and Their Potential Applications Beyond.Front Immunol. 2022 Feb 15;13:825979. doi: 10.3389/fimmu.2022.825979. eCollection 2022. Front Immunol. 2022. PMID: 35242135 Free PMC article. Review.
-
Optimizing NK-92 serial killers: gamma irradiation, CD95/Fas-ligation, and NK or LAK attack limit cytotoxic efficacy.J Transl Med. 2022 Apr 2;20(1):151. doi: 10.1186/s12967-022-03350-6. J Transl Med. 2022. PMID: 35366943 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

