Probiotic Streptococcus salivarius K12 Alleviates Radiation-Induced Oral Mucositis in Mice
- PMID: 34149727
- PMCID: PMC8213397
- DOI: 10.3389/fimmu.2021.684824
Probiotic Streptococcus salivarius K12 Alleviates Radiation-Induced Oral Mucositis in Mice
Abstract
Background: Oral mucositis is the most common oral complication of cancer patients receiving radiotherapy and/or chemotherapy, leading to poor quality of life. Limitations of the current interventions on radiation-induced oral mucositis (RIOM) urge the development of novel therapeutics. Here, we evaluated the treatment outcome of probiotic Streptococcus salivarius K12 on RIOM mice, and oral microbiota that is associated with the progress of RIOM was further investigated.
Methods: An experimental RIOM mouse model was established, and S. salivarius K12 was applied to the mouse oral cavity daily. Histological analyses were performed to evaluate the severity of oral mucositis and the treatment outcome of S. salivarius K12. The oral microbiota of mice was further analyzed by 16S rRNA sequencing, microbial culture and qPCR.
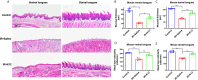
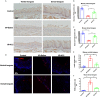
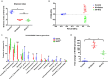
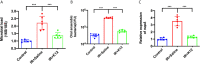
Results: Irradiation induced conspicuous mucositis in the oral cavity of mice. S. salivarius K12 treatment was beneficial for the healing of RIOM, as reflected by reduced ulcer size, increased basal layer epithelial cellularity and mucosal thickness, and elevated epithelial proliferation and attenuated apoptosis. RIOM mice presented significant oral microbial dysbiosis, with an overgrowth of oral anaerobes. S. salivarius K12 treatment reconstituted the oral microbiota and decreased the abundance of oral anaerobes of RIOM mice. In addition, S. salivarius K12 treatment inhibited NI1060 in Pasteurella genus and downregulated the expression of nitrate reductase.
Conclusions: S. salivarius K12 treatment can alleviate RIOM and reconstituted the dysbiotic oral microbiota in mice. S. salivarius K12 may represent a promising adjuvant treatment to improve the quality of life of cancer patients receiving radiotherapy.
Keywords: Streptococcus salivarius K12; dysbiosis; oral mucositis; probiotics; radiotherapy.
Copyright © 2021 Wang, Li, Zhang, Zheng, Wang, Jia, Peng, Xie, Zou, Zheng, Li, Zhou and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

