Local Respiratory Allergy: From Rhinitis Phenotype to Disease Spectrum
- PMID: 34149736
- PMCID: PMC8206788
- DOI: 10.3389/fimmu.2021.691964
Local Respiratory Allergy: From Rhinitis Phenotype to Disease Spectrum
Abstract
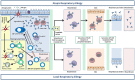
Local respiratory allergy (LRA) is defined by the negativity of atopy tests, a clinical history suggestive of airway allergy and a positive response to the nasal and/or bronchial allergen challenge. The clinical spectrum of LRA is comprised of three conditions: local allergic rhinitis (LAR) and local allergic asthma in non-atopic patients, and dual allergic rhinitis (coexistence of allergic rhinitis and LAR) in atopic individuals. LRA is an independent disease phenotype not progressing to atopy over time, but naturally evolving to the clinical worsening and the onset of comorbidities. Published data suggests that LRA is mediated through the mucosal synthesis of allergen-specific (s)IgE, which binds to FcϵRI on resident mast cells, and in >50% of cases traffics to the blood stream to sensitize circulating basophils. To date, 4 clinical trials have demonstrated the capacity of allergen immunotherapy (AIT) to decrease nasal, conjunctival and bronchial symptoms, to improve quality of life, to increase the threshold dose of allergen eliciting respiratory symptoms, and to induce serum sIgG4 in LRA individuals. Collectively, these data indicate that local allergy is a relevant disease mechanisms in both atopic and non-atopic patients with airway diseases.
Keywords: IgE synthesis; allergic rhinitis; dual allergic rhinitis; local allergic asthma; local allergic rhinitis; local respiratory allergy; mucosal immunology.
Copyright © 2021 Testera-Montes, Salas, Palomares, Ariza, Torres, Rondón and Eguiluz-Gracia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Allergen Immunotherapy for Local Respiratory Allergy.Curr Allergy Asthma Rep. 2020 May 19;20(7):23. doi: 10.1007/s11882-020-00920-w. Curr Allergy Asthma Rep. 2020. PMID: 32430550 Review.
-
Organ-specific allergen challenges in airway allergy: Current utilities and future directions.Allergy. 2023 Jul;78(7):1794-1809. doi: 10.1111/all.15731. Epub 2023 Apr 10. Allergy. 2023. PMID: 37002709 Review.
-
Local Allergic Rhinitis.J Allergy Clin Immunol Pract. 2024 Jun;12(6):1430-1433. doi: 10.1016/j.jaip.2024.04.021. Epub 2024 Apr 17. J Allergy Clin Immunol Pract. 2024. PMID: 38641133
-
Local allergic rhinitis: A critical reappraisal from a paediatric perspective.Pediatr Allergy Immunol. 2016 Sep;27(6):569-73. doi: 10.1111/pai.12577. Epub 2016 Jun 30. Pediatr Allergy Immunol. 2016. PMID: 27098888
-
IgE Test in Secretions of Patients with Respiratory Allergy.Curr Allergy Asthma Rep. 2018 Oct 13;18(12):67. doi: 10.1007/s11882-018-0821-7. Curr Allergy Asthma Rep. 2018. PMID: 30317418 Review.
Cited by
-
Neuronal-Immune Cell Units in Allergic Inflammation in the Nose.Int J Mol Sci. 2022 Jun 22;23(13):6938. doi: 10.3390/ijms23136938. Int J Mol Sci. 2022. PMID: 35805946 Free PMC article. Review.
-
The Utility of Nasal Challenges to Phenotype Asthma Patients.Int J Mol Sci. 2022 Apr 27;23(9):4838. doi: 10.3390/ijms23094838. Int J Mol Sci. 2022. PMID: 35563226 Free PMC article. Review.
-
Local allergic rhinitis: considerations.Multidiscip Respir Med. 2023 Dec 19;18(1):939. doi: 10.4081/mrm.2023.939. eCollection 2023 Jan 17. Multidiscip Respir Med. 2023. PMID: 38322132 Free PMC article.
-
Distinct Adverse Reactions to mRNA, Inactivated Virus, and Adenovirus Vector COVID-19 Vaccines: Insights from a Cohort Study on Atopic and Non-Atopic Subjects in Brazil.Vaccines (Basel). 2024 Apr 12;12(4):408. doi: 10.3390/vaccines12040408. Vaccines (Basel). 2024. PMID: 38675790 Free PMC article.
-
Clinical Practice Guideline: Immunotherapy for Inhalant Allergy.Otolaryngol Head Neck Surg. 2024 Mar;170 Suppl 1(Suppl 1):S1-S42. doi: 10.1002/ohn.648. Otolaryngol Head Neck Surg. 2024. PMID: 38408152 Free PMC article.
References
-
- Lamb CE, Ratner PH, Johnson CE, Ambegaonkar AJ, Joshi AV, Day D, et al. . Economic Impact of Workplace Productivity Losses Due to Allergic Rhinitis Compared With Select Medical Conditions in the United States From an Employer Perspective. Curr Med Res Opin (2006) 22(6):1203–10. 10.1185/030079906X112552 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

