A Methodological Advance of Tobacco Rattle Virus-Induced Gene Silencing for Functional Genomics in Plants
- PMID: 34149770
- PMCID: PMC8212136
- DOI: 10.3389/fpls.2021.671091
A Methodological Advance of Tobacco Rattle Virus-Induced Gene Silencing for Functional Genomics in Plants
Abstract
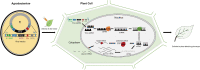
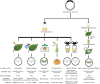
As a promising high-throughput reverse genetic tool in plants, virus-induced gene silencing (VIGS) has already begun to fulfill some of this promise in diverse aspects. However, review of the technological advancements about widely used VIGS system, tobacco rattle virus (TRV)-mediated gene silencing, needs timely updates. Hence, this article mainly reviews viral vector construction, inoculation method advances, important influential factors, and summarizes the recent applications in diverse plant species, thus providing a better understanding and advice for functional gene analysis related to crop improvements.
Keywords: TRV-VIGS; agroinfiltration; methodology modification; secondary inoculation; vector construction.
Copyright © 2021 Shi, Hao, Tian, Cao, Wei and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
High rates of virus-induced gene silencing by tobacco rattle virus in Populus.Tree Physiol. 2015 Sep;35(9):1016-29. doi: 10.1093/treephys/tpv064. Epub 2015 Jul 23. Tree Physiol. 2015. PMID: 26209619
-
California TRV-based VIGS vectors mediate gene silencing at elevated temperatures but with greater growth stunting.BMC Plant Biol. 2021 Nov 22;21(1):553. doi: 10.1186/s12870-021-03324-8. BMC Plant Biol. 2021. PMID: 34809584 Free PMC article.
-
Optimization of Tobacco Rattle Virus (TRV)-Based Virus-Induced Gene Silencing (VIGS) in Tomato.Methods Mol Biol. 2022;2408:133-145. doi: 10.1007/978-1-0716-1875-2_9. Methods Mol Biol. 2022. PMID: 35325421
-
The potential of virus-induced gene silencing for speeding up functional characterization of plant genes.Genet Mol Res. 2004 Sep 30;3(3):323-41. Genet Mol Res. 2004. PMID: 15614725 Review.
-
Virus-induced gene silencing is a versatile tool for unraveling the functional relevance of multiple abiotic-stress-responsive genes in crop plants.Front Plant Sci. 2014 Jul 8;5:323. doi: 10.3389/fpls.2014.00323. eCollection 2014. Front Plant Sci. 2014. PMID: 25071806 Free PMC article. Review.
Cited by
-
Understanding the possible influence of Pumilio RNA binding proteins on terpenoid indole alkaloid biosynthesis in Catharanthus roseus.Physiol Mol Biol Plants. 2022 May;28(5):963-969. doi: 10.1007/s12298-022-01193-5. Epub 2022 Jun 6. Physiol Mol Biol Plants. 2022. PMID: 35722510 Free PMC article.
-
LcNAC13 Is Involved in the Reactive Oxygen Species-Dependent Senescence of the Rudimentary Leaves in Litchi chinensis.Front Plant Sci. 2022 May 9;13:886131. doi: 10.3389/fpls.2022.886131. eCollection 2022. Front Plant Sci. 2022. PMID: 35615126 Free PMC article.
-
A simple and efficient gene functional analysis method for studying the growth and development of peach seedlings.Hortic Res. 2024 Jun 3;11(7):uhae155. doi: 10.1093/hr/uhae155. eCollection 2024 Jul. Hortic Res. 2024. PMID: 39005999 Free PMC article.
-
Silencing of Putative Plasmodesmata-Associated Genes PDLP and SRC2 Reveals Their Differential Involvement during Plant Infection with Cucumber Mosaic Virus.Plants (Basel). 2025 Feb 6;14(3):495. doi: 10.3390/plants14030495. Plants (Basel). 2025. PMID: 39943057 Free PMC article.
-
A New Method for Rapid Subcellular Localization and Gene Function Analysis in Cotton Based on Barley Stripe Mosaic Virus.Plants (Basel). 2022 Jul 1;11(13):1765. doi: 10.3390/plants11131765. Plants (Basel). 2022. PMID: 35807717 Free PMC article.
References
-
- Benedito V. A., Visser P. B., Angenent G. C., Krens F. A. (2004). The potential of virus-induced gene silencing for speeding up functional characterization of plant genes. Genet. Mol. Res. 3 323–341. - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

