Simultaneous reduction and adsorption of arsenite anions by green synthesis of iron nanoparticles using pomegranate peel extract
- PMID: 34150261
- PMCID: PMC8172733
- DOI: 10.1007/s40201-021-00631-y
Simultaneous reduction and adsorption of arsenite anions by green synthesis of iron nanoparticles using pomegranate peel extract
Abstract
Purpose: Arsenic is a toxic metalloid that is present in the environment as arsenate and arsenite anions. Exposure to arsenic anions caused skin problems, degenerative diseases, kidney, liver, and lung cancer. The synthesized iron nanoparticles (NPs) were examined as a green low-cost adsorbent for the removal of arsenite anions from aqueous solution via batch adsorption procedure.
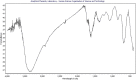
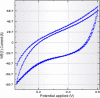
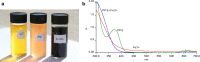
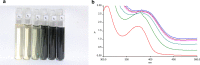
Methods: Iron NPs were prepared in a single step by the reaction of Fe+3 0.01 M solution with a fresh aqueous solution of 2% w/v pomegranate peel extract (PPE) as both reducing and capping agents. The physicochemical properties of peel were investigated by some experiments and functional groups were determined by the FT-IR spectrum. The electrochemical behavior of PPE was studied using cyclic voltammetry on a glassy carbon electrode as produced a cathodic peak at range 120-400 mV. The progress of nZVI production was monitored by a decrease of 372 nm wavelength UV-Vis spectra of PPE. The 27 adsorption experiments were carried out as a function of solution pH, initial arsenite concentration, mass adsorbent, and contact time according to DOE.
Results: The rapid rate of adsorption was observed at 20-60 min, indicating that the principal mechanism dominating the sorption process was reduction and chemical adsorption. The arsenite removal efficiency was found to be dependent on the solution pH, adsorbent dose, and initial concentration, respectively.
Conclusion: The experimental data show the ability of the synthesized iron NPs to remove arsenate from solution in both synthetic and polluted natural water. The thermodynamic study suggested the spontaneous and endothermic nature of adsorption of arsenite by green synthesized iron NPs. The iron NPs synthesized with PPE increased the removal of arsenite with an increase in the active surface, indicating some chemical interactions between the adsorbent and oxoanions.
Keywords: Arsenite oxoanions; Cyclic voltammetry; DOE; Green synthesis; Iron nanoparticles; Pomegranate peel extract.
© Springer Nature Switzerland AG 2021.
Conflict of interest statement
Conflict of interestAll authors contributed sufficiently to the study and read this final manuscript, and gave their approval for the manuscript to be submitted in the present form.
Figures


References
-
- Poursaberi T, Hassanisadi M, Nourmohammadian F. Application of synthesized nanoscale zero-valent iron in the treatment of dye solution containing basic yellow 28. Prog Color Colorants Coat. 2012;5:35–40.
-
- Shahwan T, Abu Sirriah S, Nairat M, Boyacı E, Eroğlu AE, Scott TB, Hallam KR. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem Eng J. 2011;172(1):258–266. doi: 10.1016/j.cej.2011.05.103. - DOI
-
- Yuvakkumar R, Elango V, Rajendran V, Kannan N. Preparation and characterization of zero valent iron nanoparticles. Dig J Nanomater Biostruct. 2011;6:1771–1776.
-
- Sun Y-P, Li X-Q, Zhang W-X, Wang HP. A method for the preparation of stable dispersion of zero-valent iron nanoparticles. Colloids Surf A Physicochem Eng Asp. 2007;308(1):60–66. doi: 10.1016/j.colsurfa.2007.05.029. - DOI
LinkOut - more resources
Full Text Sources

