Risk of Endometrial Cancer and Frequencies of Invasive Endometrial Procedures in Young Breast Cancer Survivors Treated With Tamoxifen: A Nationwide Study
- PMID: 34150613
- PMCID: PMC8209428
- DOI: 10.3389/fonc.2021.636378
Risk of Endometrial Cancer and Frequencies of Invasive Endometrial Procedures in Young Breast Cancer Survivors Treated With Tamoxifen: A Nationwide Study
Abstract
Background: Although the guidelines recommend gynecological assessment and close monitoring for symptoms of endometrial cancer in postmenopausal breast cancer survivors taking tamoxifen (TAM), the risk of endometrial cancer in young breast cancer survivors has not yet been fully assessed. This study aimed to investigate the risk of developing endometrial cancer and the frequencies of gynecological examinations in young breast cancer survivors taking TAM in South Korea.
Methods: A nationwide retrospective cohort study was conducted using the Health Insurance Review and Assessment Service claims data. Kaplan-Meier analyses and log-rank tests were used to assess the probability of endometrial cancer, benign endometrial conditions, and the probability of invasive endometrial procedure. To analyze the risk of endometrial cancer and benign endometrial conditions, we used a multivariable Cox proportional hazards regression model.
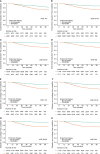
Results: Between 2010 and 2015, 60,545 newly diagnosed female breast cancer survivors were included. The total person-years were 256,099 and 140 (0.23%) patients developed endometrial cancer during the study period. In breast cancer survivors aged ≥60 years [hazard ratio (HR), 5.037; 95% confidence interval (CI), 2.185-11.613], 50-59 years (HR, 4.343; 95% CI, 2.122-8.891), and 40-49 years (HR, 2.121; 95% CI, 1.068-4.213), TAM was associated with an increased risk of endometrial cancer. In subjects aged below 40 years, TAM did not significantly increase the risk of endometrial cancer. However, among the TAM subgroups, breast cancer survivors aged below 40 years [1.61 per 1,000 person-years (PY); HR, 12.460; 95% CI, 2.698-57.522] and aged 40-49 years (2.22 per 1,000 PY; HR, 9.667; 95% CI, 4.966-18.819) with TAM-related endometrial diseases showed significantly increased risks of endometrial cancer. Among the TAM subgroup with benign endometrial conditions, the ratios of the frequency of invasive diagnostic procedures to the incidence of endometrial cancer were higher in subjects under 40 than subjects aged 60 or more.
Conclusion: Young breast cancer survivors with TAM-related benign endometrial diseases are at a higher risk of developing endometrial cancer. Gynecological surveillance should be tailored to the risk of endometrial cancer in young breast cancer survivors to improve the early detection of endometrial cancer and avoid unnecessary invasive procedures.
Keywords: breast neoplasms; dilatation and curettage; endometrial neoplasms; gynecological examination; tamoxifen.
Copyright © 2021 Choi, Lee, Jeong, Jung, Lee, Kim, Ko, Son, Ahn, Lee and Chung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J Clin Oncol (2014) 32(21):2255–69. 10.1200/JCO.2013.54.2258 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

