Blood-Based Biomarkers for Glioma in the Context of Gliomagenesis: A Systematic Review
- PMID: 34150629
- PMCID: PMC8211985
- DOI: 10.3389/fonc.2021.665235
Blood-Based Biomarkers for Glioma in the Context of Gliomagenesis: A Systematic Review
Abstract
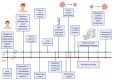
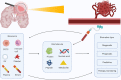
Background: Gliomas are the most common and aggressive tumors of the central nervous system. A robust and widely used blood-based biomarker for glioma has not yet been identified. In recent years, a plethora of new research on blood-based biomarkers for glial tumors has been published. In this review, we question which molecules, including proteins, nucleic acids, circulating cells, and metabolomics, are most promising blood-based biomarkers for glioma diagnosis, prognosis, monitoring and other purposes, and align them to the seminal processes of cancer.
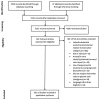
Methods: The Pubmed and Embase databases were systematically searched. Biomarkers were categorized in the identified biomolecules and biosources. Biomarker characteristics were assessed using the area under the curve (AUC), accuracy, sensitivity and/or specificity values and the degree of statistical significance among the assessed clinical groups was reported.
Results: 7,919 references were identified: 3,596 in PubMed and 4,323 in Embase. Following screening of titles, abstracts and availability of full-text, 262 articles were included in the final systematic review. Panels of multiple biomarkers together consistently reached AUCs >0.8 and accuracies >80% for various purposes but especially for diagnostics. The accuracy of single biomarkers, consisting of only one measurement, was far more variable, but single microRNAs and proteins are generally more promising as compared to other biomarker types.
Conclusion: Panels of microRNAs and proteins are most promising biomarkers, while single biomarkers such as GFAP, IL-10 and individual miRNAs also hold promise. It is possible that panels are more accurate once these are involved in different, complementary cancer-related molecular pathways, because not all pathways may be dysregulated in cancer patients. As biomarkers seem to be increasingly dysregulated in patients with short survival, higher tumor grades and more pathological tumor types, it can be hypothesized that more pathways are dysregulated as the degree of malignancy of the glial tumor increases. Despite, none of the biomarkers found in the literature search seem to be currently ready for clinical implementation, and most of the studies report only preliminary application of the identified biomarkers. Hence, large-scale validation of currently identified and potential novel biomarkers to show clinical utility is warranted.
Keywords: blood; diagnostics; glioblastoma; glioma; liquid biopsy.
Copyright © 2021 Ali, Harting, de Vries, Ali, Wurdinger and Best.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

