Organ-Specific Branching Morphogenesis
- PMID: 34150767
- PMCID: PMC8212048
- DOI: 10.3389/fcell.2021.671402
Organ-Specific Branching Morphogenesis
Abstract
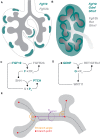
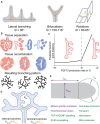
A common developmental process, called branching morphogenesis, generates the epithelial trees in a variety of organs, including the lungs, kidneys, and glands. How branching morphogenesis can create epithelial architectures of very different shapes and functions remains elusive. In this review, we compare branching morphogenesis and its regulation in lungs and kidneys and discuss the role of signaling pathways, the mesenchyme, the extracellular matrix, and the cytoskeleton as potential organ-specific determinants of branch position, orientation, and shape. Identifying the determinants of branch and organ shape and their adaptation in different organs may reveal how a highly conserved developmental process can be adapted to different structural and functional frameworks and should provide important insights into epithelial morphogenesis and developmental disorders.
Keywords: branch angle; branch distance; branch shape; branching morphogenesis; kidney; lung; tissue mechanics; turing pattern.
Copyright © 2021 Lang, Conrad and Iber.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

