Roles of Melatonin in Goat Hair Follicle Stem Cell Proliferation and Pluripotency Through Regulating the Wnt Signaling Pathway
- PMID: 34150780
- PMCID: PMC8212062
- DOI: 10.3389/fcell.2021.686805
Roles of Melatonin in Goat Hair Follicle Stem Cell Proliferation and Pluripotency Through Regulating the Wnt Signaling Pathway
Abstract
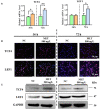
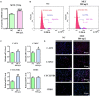
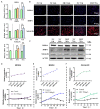
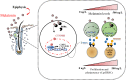
Emerging studies show that melatonin promotes cashmere development through hypodermic implantation. However, the impact and underlying mechanisms are currently unknown. In vitro study has previously demonstrated that melatonin induces cashmere growth by regulating the proliferation of goat secondary hair follicle stem cells (gsHFSCs), but there is limited information concerning the effects of melatonin on cell pluripotency. It is also known that Wnt signaling may actively participate in regulating cell proliferation and stem cell pluripotency. Therefore, in the current investigation, goat hair follicle stem cells were exposed to multiple concentrations of melatonin and different culture times to reveal the relationship between melatonin and the activation of Wnt signaling. A proportionally high Catenin beta-1 (CTNNB1) response was induced by 500 ng/L of melatonin, but it was then suppressed with the dosages over 1,000 ng/L. Greater amounts of CTNNB1 entered the cell nuclei by extending the exposure time to 72 h, which activated transcription factor 4/lymphoid enhancer-binding factor 1 and promoted the expression of the proliferation-related genes C-MYC, C-JUN, and CYCLIND1. Moreover, nuclear receptor ROR-alpha (RORα) and bone morphogenetic protein 4 (BMP4) were employed to analyze the underlying mechanism. RORα presented a sluggish concentration/time-dependent rise, but BMP4 was increased dramatically by melatonin exposure, which revealed that melatonin might participate in regulating the pluripotency of hair follicle stem cells. Interestingly, NOGGIN, which is a BMP antagonist and highly relevant to cell stemness, was also stimulated by melatonin. These findings demonstrated that melatonin exposure and/or NOGGIN overexpression in hair follicle stem cells might promote the expression of pluripotency markers Homeobox protein NANOG, Organic cation/carnitine transporter 4, and Hematopoietic progenitor cell antigen CD34. Our findings here provided a comprehensive view of Wnt signaling in melatonin stimulated cells and melatonin mediated stemness of gsHFSCs by regulating NOGGIN, which demonstrates a regulatory mechanism of melatonin enhancement on the growth of cashmere.
Keywords: CTNNB1; Wnt pathway; cashmere; hair follicle stem cells; melatonin.
Copyright © 2021 Zhang, Wang, Zhang, Wang, Ge and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Melatonin promotes Cashmere goat (Capra hircus) secondary hair follicle growth: a view from integrated analysis of long non-coding and coding RNAs.Cell Cycle. 2018;17(10):1255-1267. doi: 10.1080/15384101.2018.1471318. Epub 2018 Jul 17. Cell Cycle. 2018. PMID: 29895193 Free PMC article.
-
Time-course RNA-seq analysis reveals stage-specific and melatonin-triggered gene expression patterns during the hair follicle growth cycle in Capra hircus.BMC Genomics. 2022 Feb 16;23(1):140. doi: 10.1186/s12864-022-08331-z. BMC Genomics. 2022. PMID: 35172715 Free PMC article.
-
Efficient establishment of an optimized culture condition for cashmere goat primary hair follicle stem cells.J Anim Sci. 2023 Jan 3;101:skad235. doi: 10.1093/jas/skad235. J Anim Sci. 2023. PMID: 37429584 Free PMC article.
-
Melatonin's Role in Hair Follicle Growth and Development: A Cashmere Goat Perspective.Int J Mol Sci. 2025 Mar 21;26(7):2844. doi: 10.3390/ijms26072844. Int J Mol Sci. 2025. PMID: 40243438 Free PMC article. Review.
-
Melatonin and the hair follicle.J Pineal Res. 2008 Jan;44(1):1-15. doi: 10.1111/j.1600-079X.2007.00512.x. J Pineal Res. 2008. PMID: 18078443 Review.
Cited by
-
Identification of lncRNAs involved in the hair follicle cycle transition of cashmere goats in response to photoperiod change.BMC Genomics. 2025 May 15;26(1):487. doi: 10.1186/s12864-025-11675-x. BMC Genomics. 2025. PMID: 40375123 Free PMC article.
-
REC8 enhances stemness and promotes metastasis of colorectal cancer through BTK/Akt/β-catenin signaling pathway.Transl Oncol. 2022 Jan;15(1):101305. doi: 10.1016/j.tranon.2021.101305. Epub 2021 Dec 7. Transl Oncol. 2022. PMID: 34890967 Free PMC article.
-
Melatonin's effect on hair follicles in a goat (Capra hircus) animal model.Front Endocrinol (Lausanne). 2024 Apr 2;15:1361100. doi: 10.3389/fendo.2024.1361100. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38628581 Free PMC article.
-
Multi-omics and AI-driven advances in miRNA-mediated hair follicle regulation in cashmere goats.Front Vet Sci. 2025 Jul 9;12:1635202. doi: 10.3389/fvets.2025.1635202. eCollection 2025. Front Vet Sci. 2025. PMID: 40703926 Free PMC article. Review.
-
Whole-transcriptome RNA sequencing reveals global expression dynamics and ceRNA regulatory networks related to hair follicle development and melanogenesis in goats.Anim Biosci. 2025 Sep;38(9):1841-1857. doi: 10.5713/ab.24.0617. Epub 2025 Mar 31. Anim Biosci. 2025. PMID: 40211852 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

