Management of Chronic Hyperkalemia in Patients With Chronic Kidney Disease: An Old Problem With News Options
- PMID: 34150795
- PMCID: PMC8213200
- DOI: 10.3389/fmed.2021.653634
Management of Chronic Hyperkalemia in Patients With Chronic Kidney Disease: An Old Problem With News Options
Abstract
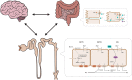
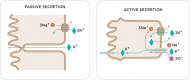
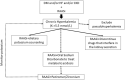
Hyperkalemia is one of the main electrolyte disorders in patients with chronic kidney disease (CKD). The prevalence of hyperkalemia increases as the Glomerular Filtration Rate (GFR) declines. Although chronic hyperkalemia is not a medical emergency, it can have negative consequences for the adequate cardio-renal management in the medium and long term. Hyperkalemia is common in patients on renin-angiotensin-aldosterone system inhibitors (RAASi) or Mineralocorticoid Receptor Antagonists (MRAs) and can affect treatment optimization for hypertension, diabetes mellitus, heart failure (HF), and CKD. Mortality rates are higher with suboptimal dosing among patients with CKD, diabetes or HF compared with full RAASi dosing, and are the highest among patients who discontinue RAASis. The treatment of chronic hyperkalemia is still challenging. Therefore, in the real world, discontinuation or reduction of RAASi therapy may lead to adverse cardiorenal outcomes, and current guidelines differ with regard to recommendations on RAASi therapy to enhance cardio and reno-protective effects. Treatment options for hyperkalemia have not changed much since the introduction of the cation exchange resin over 50 years ago. Nowadays, two new potassium binders, Patiromer Sorbitex Calcium, and Sodium Zirconium Cyclosilicate (SZC) already approved by FDA and by the European Medicines Agency, have demonstrated their clinical efficacy in reducing serum potassium with a good safety profile. The use of the newer potassium binders may allow continuing and optimizing RAASi therapy in patients with hyperkalemia keeping the cardio-renal protective effect in patients with CKD and cardiovascular disease. However, further research is needed to address some questions related to potassium disorders (definition of chronic hyperkalemia, monitoring strategies, prediction score for hyperkalemia or length for treatment).
Keywords: RAASi; chronic kidney disease; hyperkalemia; patiromer; potassium binders; zirconium.
Copyright © 2021 Morales, Cravedi and Manrique.
Conflict of interest statement
EM and JM report serving as consultants participating in advisory boards for Vifor Pharma Group Company. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

