Mesoporous Biopolymer Architecture Enhanced the Adsorption and Selectivity of Aqueous Heavy-Metal Ions
- PMID: 34151111
- PMCID: PMC8210456
- DOI: 10.1021/acsomega.1c01642
Mesoporous Biopolymer Architecture Enhanced the Adsorption and Selectivity of Aqueous Heavy-Metal Ions
Abstract
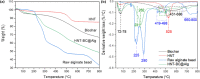
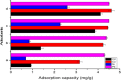
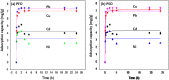
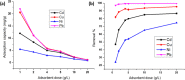
Halloysite nanotubes (HNT) and ball-milled biochar (BC) incorporated biocompatible mesoporous adsorbents (HNT-BC@Alg) were synthesized for adsorption of aqueous heavy-metal ions. HNT-BC@Alg outperformed the BC, HNT, and BC@Alg in removing cadmium (Cd), copper (Cu), nickel (Ni), and lead (Pb). Mesoporous structure (∼7.19 to 7.56 nm) of HNT-BC@Alg was developed containing an abundance of functional groups induced from encapsulated BC and tubular HNT, which allowed heavy metals to infiltrate and interact with the adsorbents. Siloxane groups from HNT, oxygen-containing functional groups from BC, and hydroxyl and carboxyl groups from alginate polymer play a significant role in the adsorption of heavy-metal ions. The removal percentage of heavy metals was recorded as Pb (∼99.97 to 99.05%) > Cu (∼95.01 to 90.53%) > Cd (∼92.5 to 55.25%) > Ni (∼80.85 to 50.6%), even in the presence of 0.01/0.001 M of CaCl2 and Na2SO4 as background electrolytes and charged organic molecule under an environmentally relevant concentration (200 μg/L). The maximum adsorption capacities of Ni, Cd, Cu, and Pb were calculated as 2.85 ± 0.08, 6.96 ± 0.31, 16.87 ± 1.50, and 26.49 ± 2.04 mg/g, respectively. HNT-BC@Alg has fast sorption kinetics and maximum adsorption capacity within a short contact time (∼2 h). Energy-dispersive X-ray spectroscopy (EDS) elemental mapping exhibited that adsorbed heavy metals co-distributed with Ca, Si, and Al. The reduction of surface area, pore volume, and pore area of HNT-BC@Alg (after sorption of heavy metals) confirms that mesoporous surface (2-18 nm) supports diffusion, infiltration, and interaction. However, a lower range of mesoporous diameter of the adsorbent is more suitable for the adsorption of heavy-metal ions. The adsorption isotherm and kinetics fitted well with the Langmuir isotherm and the pseudo-second-order kinetic models, demonstrating the monolayer formation of heavy-metal ions through both the physical sorption and chemical sorption, including pore filling, ion exchange, and electrostatic interaction.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




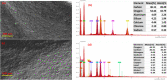



References
-
- Basheer A. A. New generation nano-adsorbents for the removal of emerging contaminants in water. J. Mol. Liq. 2018, 261, 583–593. 10.1016/j.molliq.2018.04.021. - DOI
-
- Ali I.; Jain C. K. Groundwater contamination and health hazards by some of the most commonly used pesticides. Curr. Sci. 1998, 75, 1011–1014.
-
- Ahsan M. A.; Jabbari V.; Imam M. A.; Castro E.; Kim H.; Curry M. L.; Valles-Rosales D. J.; Noveron J. C. Nanoscale nickel metal organic framework decorated over graphene oxide and carbon nanotubes for water remediation. Sci. Total Environ. 2020, 698, 13421410.1016/j.scitotenv.2019.134214. - DOI - PubMed
LinkOut - more resources
Full Text Sources

