Functional Selectivity of a Biased Cannabinoid-1 Receptor (CB1R) Antagonist
- PMID: 34151207
- PMCID: PMC8204328
- DOI: 10.1021/acsptsci.1c00048
Functional Selectivity of a Biased Cannabinoid-1 Receptor (CB1R) Antagonist
Abstract
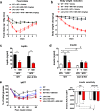
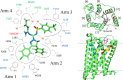
Seven-transmembrane receptors signal via G-protein- and β-arrestin-dependent pathways. We describe a peripheral CB1R antagonist (MRI-1891) highly biased toward inhibiting CB1R-induced β-arrestin-2 (βArr2) recruitment over G-protein activation. In obese wild-type and βArr2-knockout (KO) mice, MRI-1891 treatment reduces food intake and body weight without eliciting anxiety even at a high dose causing partial brain CB1R occupancy. By contrast, the unbiased global CB1R antagonist rimonabant elicits anxiety in both strains, indicating no βArr2 involvement. Interestingly, obesity-induced muscle insulin resistance is improved by MRI-1891 in wild-type but not in βArr2-KO mice. In C2C12 myoblasts, CB1R activation suppresses insulin-induced akt-2 phosphorylation, preventable by MRI-1891, βArr2 knockdown or overexpression of CB1R-interacting protein. MRI-1891, but not rimonabant, interacts with nonpolar residues on the N-terminal loop, including F108, and on transmembrane helix-1, including S123, a combination that facilitates βArr2 bias. Thus, CB1R promotes muscle insulin resistance via βArr2 signaling, selectively mitigated by a biased CB1R antagonist at reduced risk of central nervous system (CNS) side effects.
Not subject to U.S. Copyright. Published 2021 by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): G.K., R.C., and M.R.I. are listed as co-inventors on two US Government patents covering (S)-MRI-1891. The remaining authors declare no competing interests.
Figures







References
-
- Van Gaal L. F.; Rissanen A. M.; Scheen A. J.; Ziegler O.; Rossner S. (2005) Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365 (9468), 1389–1397. 10.1016/S0140-6736(05)66374-X. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
