Capacity for increased surface area in the hydrophobic core of β-sheet peptide bilayer nanoribbons
- PMID: 34151480
- PMCID: PMC8349901
- DOI: 10.1002/psc.3334
Capacity for increased surface area in the hydrophobic core of β-sheet peptide bilayer nanoribbons
Abstract
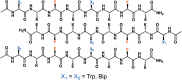
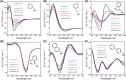
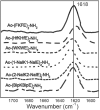
Amphipathic peptides with amino acids arranged in alternating patterns of hydrophobic and hydrophilic residues efficiently self-assemble into β-sheet bilayer nanoribbons. Hydrophobic side chain functionality is effectively buried in the interior of the putative bilayer of these nanoribbons. This study investigates consequences on self-assembly of increasing the surface area of aromatic side chain groups that reside in the hydrophobic core of nanoribbons derived from Ac-(XKXE)2 -NH2 peptides (X = hydrophobic residue). A series of Ac-(XKXE)2 -NH2 peptides incorporating aromatic amino acids of increasing molecular volume and steric profile (X = phenylalanine [Phe], homophenylalanine [Hph], tryptophan [Trp], 1-naphthylalanine [1-Nal], 2-naphthylalanine [2-Nal], or biphenylalanine [Bip]) were assessed to determine substitution effects on self-assembly propensity and on morphology of the resulting nanoribbon structures. Additional studies were conducted to determine the effects of incorporating amino acids of differing steric profile in the hydrophobic core (Ac-X1 KFEFKFE-NH2 and Ac-(X1,5 KFE)-NH2 peptides, X = Trp or Bip). Spectroscopic analysis by circular dichroism (CD) and Fourier transform infrared (FT-IR) spectroscopy indicated β-sheet formation for all variants. Self-assembly rate increased with peptide hydrophobicity; increased molecular volume of the hydrophobic side chain groups did not appear to induce kinetic penalties on self-assembly rates. Transmission electron microscopy (TEM) imaging indicated variation in fibril morphology as a function of amino acid in the X positions. This study confirms that hydrophobicity of amphipathic Ac-(XKXE)2 -NH2 peptides correlates to self-assembly propensity and that the hydrophobic core of the resulting nanoribbon bilayers has a significant capacity to accommodate sterically demanding functional groups. These findings provide insight that may be used to guide the exploitation of self-assembled amphipathic peptides as functional biomaterials.
Keywords: amphipathic; nanoribbons; non-natural amino acids; self-assembling peptides; β-sheet fibrils.
© 2021 The Authors. Journal of Peptide Science published by European Peptide Society and John Wiley & Sons Ltd.
Figures






References
-
- Galler KM, D'Souza RN, Hartgerink JD. Biomaterials and their potential applications for dental tissue engineering. J Mater Chem. 2010;20(40):8730‐8746. 10.1039/c0jm01207f - DOI
MeSH terms
Substances
Grants and funding
- R25 GM127199/GM/NIGMS NIH HHS/United States
- U54 MD012388/MD/NIMHD NIH HHS/United States
- U54 CA143925/CA/NCI NIH HHS/United States
- CHE-0946653/U.S. National Science Foundation
- CHE-0840410/U.S. National Science Foundation
- T32 GM118283/GM/NIGMS NIH HHS/United States
- 1U54MD012388-01/MD/NIMHD NIH HHS/United States
- 2U54CA143925-11/CA/NCI NIH HHS/United States
- R25GM127199/GM/NIGMS NIH HHS/United States
- CHE-1904528/National Science Foundation
- T32 GM145461/GM/NIGMS NIH HHS/United States
- DMR-1148836/National Science Foundation
- R01 HL138538/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
