Differential localization patterns of claudin 10, 16, and 19 in human, mouse, and rat renal tubular epithelia
- PMID: 34151590
- PMCID: PMC8424667
- DOI: 10.1152/ajprenal.00579.2020
Differential localization patterns of claudin 10, 16, and 19 in human, mouse, and rat renal tubular epithelia
Abstract
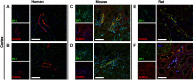
Functional properties of the paracellular pathway depend critically on the set of claudins (CLDN) expressed at the tight junction. Two syndromes are causally linked to loss-of-function mutations of claudins: hypohidrosis, electrolyte imbalance, lacrimal gland dysfunction, ichthyosis, and xerostomia (HELIX) syndrome caused by genetic variations in the CLDN10 gene and familial hypomagnesemia with hypercalciuria and nephrocalcinosis caused by genetic variations in the CLDN16 or CLDN19 genes. All three genes are expressed in the kidney, particularly in the thick ascending limb (TAL). However, localization of these claudins in humans and rodents remains to be delineated in detail. We studied the segmental and subcellular expression of CLDN10, CLDN16, and CLDN19 in both paraffin-embedded and frozen kidney sections from the adult human, mouse, and rat using immunohistochemistry and immunofluorescence, respectively. Here, CLDN10 was present in a subset of medullary and cortical TAL cells, localizing to basolateral domains and tight junctions in human and rodent kidneys. Weak expression was detected at the tight junction of proximal tubular cells. CLDN16 was primarily expressed in a subset of TAL cells in the cortex and outer stripe of outer medulla, restricted to basolateral domains and tight junctional structures in both human and rodent kidneys. CLDN19 predominantly colocalized with CLDN16 in tight junctions and basolateral domains of the TAL but was also found in basolateral and junctional domains in more distal sites. CLDN10 expression at tight junctions almost never overlapped with that of CLND16 and CLDN19, consistent with distinct junctional pathways with different permeation profiles in both human and rodent kidneys.NEW & NOTEWORTHY This study used immunohistochemistry and immunofluorescence to investigate the distribution of claudin 10, 16, and 19 in the human, mouse, and rat kidney. The findings showed distinct junctional pathways in both human and rodent kidneys, supporting the existence of different permeation profiles in all species investigated.
Keywords: epithelium; immunolocalization; kidney; thick ascending limb; tight junction.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures








References
-
- Himmerkus N, Plain A, Marques RD, Sonntag SR, Paliege A, Leipziger J, Bleich M. AVP dynamically increases paracellular Na+ permeability and transcellular NaCl transport in the medullary thick ascending limb of Henle’s loop. Pflugers Arch 469: 149–158, 2017. doi:10.1007/s00424-016-1915-5. - DOI - PubMed
-
- Loupy A, Ramakrishnan SK, Wootla B, Chambrey R, de la Faille R, Bourgeois S, Bruneval P, Mandet C, Christensen EI, Faure H, Cheval L, Laghmani K, Collet C, Eladari D, Dodd RH, Ruat M, Houillier P. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest 122: 3355–3367, 2012. doi:10.1172/JCI57407. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

