Effect of Age on Clinical Trial Outcome in Participants with Probable Alzheimer's Disease
- PMID: 34151817
- PMCID: PMC8461716
- DOI: 10.3233/JAD-210530
Effect of Age on Clinical Trial Outcome in Participants with Probable Alzheimer's Disease
Abstract
Background: Age may affect treatment outcome in trials of mild probable Alzheimer's disease (AD).
Objective: We examined age as a moderator of outcome in an exploratory study of deep brain stimulation targeting the fornix (DBS-f) region in participants with AD.
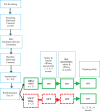
Methods: Forty-two participants were implanted with DBS electrodes and randomized to double-blind DBS-f stimulation ("on") or sham DBS-f ("off") for 12 months.
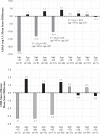
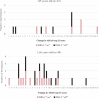
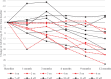
Results: The intervention was safe and well tolerated. However, the selected clinical measures did not differentiate between the "on" and "off" groups in the intent to treat (ITT) population. There was a significant age by time interaction with the Alzheimer's Disease Assessment Scale; ADAS-cog-13 (p = 0.028). Six of the 12 enrolled participants < 65 years old (50%) markedly declined on the ADAS-cog-13 versus only 6.7%of the 30 participants≥65 years old regardless of treatment assignment (p = 0.005). While not significant, post-hoc analyses favored DBS-f "off" versus "on" over 12 months in the < 65 age group but favored DBS-f "on" versus "off" in the≥65 age group on all clinical metrics. On the integrated Alzheimer's Disease rating scale (iADRS), the effect size contrasting DBS-f "on" versus "off" changed from +0.2 (favoring "off") in the < 65 group to -0.52 (favoring "on") in the≥65 age group.
Conclusion: The findings highlight issues with subject selection in clinical trials for AD. Faster disease progression in younger AD participants with different AD sub-types may influence the results. Biomarker confirmation and genotyping to differentiate AD subtypes is important for future clinical trials.
Keywords: Age; Alzheimer’s disease; clinical trials; deep brain stimulation; subject selection.
Conflict of interest statement
Authors’ disclosures available online (
Figures

References
-
- Kim EJ, Cho SS, Jeong Y, Park KC, Kang SJ, Kang E, Kim SE, Lee KH, Na DL (2005) Glucose metabolism in early onset versus late onset Alzheimer’s disease: An SPM analysis of 120 participants. Brain 128, 1790–1801. - PubMed
-
- van der Vlies AE, Koedam EL, Pijnenburg YA, Twisk JW, Scheltens P, van der Flier WM (2009) Most rapid cognitive decline in APOE e4 negative Alzheimer’s disease with early onset. Psychol Med 39, 1907–1911. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

