LncRNA BCAR4 expression predicts the clinical response to neoadjuvant chemotherapy in patients with locally advanced breast cancer
- PMID: 34151842
- PMCID: PMC8673519
- DOI: 10.3233/CBM-210048
LncRNA BCAR4 expression predicts the clinical response to neoadjuvant chemotherapy in patients with locally advanced breast cancer
Abstract
Background: Neoadjuvant chemotherapy (NAC) is an important treatment for locally advanced breast cancer (LABC). However, there are no effective biomarkers to predict the efficacy. Therefore, there is an urgent need for new biomarkers to predict the response of LABC to NAC. LncRNA BCAR4 has been detected in a variety of malignant tumor tissues and used as a new biomarker for diagnosis and prognosis. However, LncRNA BCAR4 predicts the response of LABC to NAC is unclear.
Objective: Explore the predictive effect of LncRNA BCAR4 on the efficacy of NAC for LABC in three different evaluation systems.
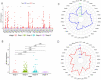
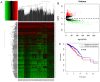
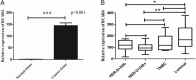
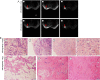
Methods: First, the TCGA database was used to analyze the expression of LncRNA BCAR4 in 33 kinds of malignant tumors, and further explore its expression in breast cancer and its impact on the survival and prognosis of breast cancer. Furthermore, quantitative methods were used to measure the expression level of LncRNA BCAR4 in cancer tissues of 48 LABC patients, and the correlation between LncRNA BCAR4 and clinicopathological status and response to NAC under the evaluation system of 3, RECIST1.1, Miller-Payne (MP) score and whether it reaches pCR,was analyzed.
Results: TCGA data analysis found that LncRNA is highly expressed in a variety of malignant tumor tissues, including breast cancer. And relatively low expression, the shorter the overall survival time of high expression patients. The high expression of LncRNA BCAR4 is related to the size of the tumor, and there are differences in expression between stage I and other stages, but there is no obvious correlation with the positive lymph node and hormone receptor status. Among the three evaluation systems, only in the RECIST 1.1 evaluation system LncRNA BCAR4 has a predictive effect on NAC for LABC. The expression of LncRNA BCAR4 has no significant correlation with clinical stage, Ki-67% and hormone receptor status, and has no significant correlation with whether patients with locally advanced breast cancer obtain pCR during neoadjuvant chemotherapy.
Conclusion: LncRNA BCAR4 is highly expressed in LABC tissues and may be an effective marker for predicting the efficacy of NAC for LABC.
Keywords: LncRNA BCAR4; Locally advanced breast cancer; clinical complete response; neoadjuvant chemotherapy; pathological complete response.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J Clin 70 (2020), 313. - PubMed
-
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P., Taylor C., Correa C., Cutter D., Duane F., Ewertz M., Gray R., Mannu G., Peto R., Whelan T., Wang Y., Wang Z. and Darby S., Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials, Lancet 383 (2014), 2127–2135. - PMC - PubMed
-
- Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., Bonnefoi H., Cameron D., Gianni L., Valagussa P., Swain S.M., Prowell T., Loibl S., Wickerham D.L., Bogaerts J., Baselga J., Perou C., Blumenthal G., Blohmer J., Mamounas E.P., Bergh J., Semiglazov V., Justice R., Eidtmann H., Paik S., Piccart M., Sridhara R., Fasching P.A., Slaets L., Tang S., Gerber B., Geyer C.E., Jr, Pazdur R., Ditsch N., Rastogi P., Eiermann W. and von Minckwitz G., Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis, Lancet 384 (2014), 164–172. - PubMed
-
- Tanioka M., Fan C., Parker J.S., Hoadley K.A., Hu Z., Li Y., Hyslop T.M., Pitcher B.N., Soloway M.G., Spears P.A., Henry L.N., Tolaney S., Dang C.T., Krop I.E., Harris L.N., Berry D.A., Mardis E.R., Winer E.P., Hudis C.A., Carey L.A. and Perou C.M., Integrated analysis of RNA and DNA from the phase III trial CALGB 40601 identifies predictors of response to trastuzumab-based neoadjuvant chemotherapy in HER2-Positive breast cancer, Clin Cancer Res 24 (2018), 5292–5304. - PMC - PubMed
-
- Fumagalli D., Venet D., Ignatiadis M., H.A. Azim Jr, Maetens M., Roth F., Salgado R., Bradbury I., Pusztai L., Harbeck N., Gomez H., Chang T.W., Coccia-Portugal M.A., Di Cosimo S., de Azambuja E., de la Pea L., Nuciforo P., Brase J.C., Huober J., Baselga J., Piccart M., Loi S. and Sotiriou C., RNA sequencing to predict response to neoadjuvant Anti-HER2 therapy: A secondary analysis of the NeoALTTO randomized clinical trial, JAMA Oncol 3 (2017), 227–234. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

