Validation of the Sysmex XN-V hematology analyzer for canine specimens
- PMID: 34152026
- PMCID: PMC8362000
- DOI: 10.1111/vcp.12936
Validation of the Sysmex XN-V hematology analyzer for canine specimens
Abstract
Background: The Sysmex XN-V is derived from the new Sysmex XN series of human hematology analyzers. The main changes from the previously validated XT-2000iV analyzer include an optic-fluorescent analysis for platelets and nucleated RBC count.
Objective: We aimed to validate the Sysmex XN-V for canine blood according to American College for Veterinary Clinical Pathology and International Council for Standardization in Hematology recommendations.
Materials and methods: Canine EDTA blood specimens and quality control material were analyzed on the Sysmex XN-V to evaluate imprecision, bias, linearity, a comparison with the XT-2000iV analyzer, interference effects, carry-over, and stability. We also verified previously established Sysmex XT-2000iV reference intervals (RIs).
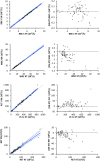
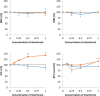
Results: Imprecision and bias were low (<5%) for most variables. Observed total error was lower than allowable total error for most measured variables except lymphocytes and monocytes. Visually determined linearity was excellent for all variables, except for lymphocytes. The correlation between the XN-V and XT-2000iV analyzers was high (>0.93) for all variables except MCHC and reticulocyte indices. Correlations between the Sysmex XN-V and manual differential counts were good for neutrophils and eosinophils, acceptable for lymphocytes, and fair for monocytes. Hemolysis, lipemia, and to a lesser extent icterus, had significant effects on measured hemoglobin concentration and associated variables. Carry-over was not visually observed for any variable. Changes in the Sysmex XN-V measurements after storage at 4℃ and 24℃ were similar to those described for the Sysmex XT-2000iV analyzer. The previously established Sysmex XT-2000iV RIs can be used to interpret results from the Sysmex XN-V analyzer for most variables except red blood cell distribution width and mean platelet volume.
Conclusions: The performance of the Sysmex XN-V analyzer was excellent and compared favorably with the Sysmex XT-2000iV analyzer.
Keywords: blood; comparison study; dog; method validation.
© 2021 The Authors. Veterinary Clinical Pathology published by Wiley Periodicals LLC on behalf of American Society for Veterinary Clinical Pathology.
Conflict of interest statement
The authors do not have any conflict of interest to disclose.
Figures




Similar articles
-
Validation of the Sysmex XN-V hematology analyzer for feline specimens.Vet Clin Pathol. 2024 Sep;53(3):294-308. doi: 10.1111/vcp.13377. Epub 2024 Sep 18. Vet Clin Pathol. 2024. PMID: 39294107
-
Evaluation of the automated hematology analyzer Sysmex XT-2000iV ™ compared to the ADVIA ® 2120 for its use in dogs, cats, and horses. Part II: Accuracy of leukocyte differential and reticulocyte count, impact of anticoagulant and sample aging.J Vet Diagn Invest. 2012 Jan;24(1):74-89. doi: 10.1177/1040638711436243. J Vet Diagn Invest. 2012. PMID: 22362937
-
Evaluation of blood erythroid parameters in male broiler chickens (Ross 308) with the Sysmex XT-2000iV and Sysmex XN-1000V analyzers and determination of hematological reference intervals obtained with manual and instrumental methods.Vet Clin Pathol. 2025 Jun;54(2):106-119. doi: 10.1111/vcp.70009. Epub 2025 Jun 3. Vet Clin Pathol. 2025. PMID: 40462459 Free PMC article.
-
Evaluation of canine and feline leukocyte differential counts obtained with the scil vCell 5 compared to the Advia 2120 hematology analyzer and a manual method.J Vet Diagn Invest. 2023 Nov;35(6):679-697. doi: 10.1177/10406387231187899. Epub 2023 Aug 23. J Vet Diagn Invest. 2023. PMID: 37612877 Free PMC article. Review.
-
Diagnostic role of Sysmex hematology analyzer in the detection of malaria: A systematic review and meta-analysis.PLoS One. 2024 Sep 6;19(9):e0296766. doi: 10.1371/journal.pone.0296766. eCollection 2024. PLoS One. 2024. PMID: 39240990 Free PMC article.
Cited by
-
Characterization of Feline Basophils on the Sysmex XN-1000V and Evaluation of a New WDF Gating Profile.Animals (Basel). 2024 Nov 22;14(23):3362. doi: 10.3390/ani14233362. Animals (Basel). 2024. PMID: 39682327 Free PMC article.
-
Validation of the Sysmex XN-V Automated Nucleated Red Blood Cell Enumeration for Canine and Feline EDTA-Anticoagulated Blood.Animals (Basel). 2024 Jan 30;14(3):455. doi: 10.3390/ani14030455. Animals (Basel). 2024. PMID: 38338098 Free PMC article.
-
Exploring frailty in apparently healthy senior dogs: a cross-sectional study.BMC Vet Res. 2024 Sep 28;20(1):436. doi: 10.1186/s12917-024-04296-1. BMC Vet Res. 2024. PMID: 39342207 Free PMC article.
-
Evaluation of the scil vCell 5, a novel laser- and impedance-based point-of-care hematology analyzer, for use in dogs and cats.J Vet Diagn Invest. 2022 May;34(3):504-517. doi: 10.1177/10406387221083621. Epub 2022 Mar 24. J Vet Diagn Invest. 2022. PMID: 35331075 Free PMC article.
-
Spurious laboratory results associated with immunoglobulin M gammopathy in a dog with multiple myeloma.J Vet Intern Med. 2022 Nov;36(6):2181-2186. doi: 10.1111/jvim.16540. Epub 2022 Sep 20. J Vet Intern Med. 2022. PMID: 36125290 Free PMC article.
References
-
- Lilliehook I, Tvedten H. Validation of the Sysmex XT‐2000iV hematology system for dogs, cats, and horses. I. Erythrocytes, platelets, and total leukocyte counts. Vet Clin Pathol. 2009;38:163‐174. - PubMed
-
- Lilliehook I, Tvedten H. Validation of the Sysmex XT‐2000iV hematology system for dogs, cats, and horses. II. Differential leukocyte counts. Vet Clin Pathol. 2009;38:175‐182. - PubMed
-
- Arnold JE, Camus MS, Freeman KP, et al. ASVCP guidelines: principles of quality assurance and standards for veterinary clinical pathology (version 3.0): developed by the American Society for Veterinary Clinical Pathology's (ASVCP) Quality Assurance and Laboratory Standards (QALS) Committee. Vet Clin Pathol. 2019;48:542‐618. - PubMed
-
- Friedrichs K, Barnhart K, Blanco J, et al. ASVCP Quality Assurance and Laboratory Standards Committee (QALS). Guidelines for the Determination of Reference Intervals in Veterinary Species and other related topics: SCOPE https://cdn.ymaws.com/www.asvcp.org/resource/resmgr/QALS/Other_Publicati.... date assessed: April 2020.
-
- Jensen AL, Kjelgaard‐Hansen M. Method comparison in the clinical laboratory. Vet Clin Pathol. 2006;35:276‐286. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

