Homogeneity or heterogeneity, the paradox of neurovascular pericytes in the brain
- PMID: 34152032
- PMCID: PMC8453512
- DOI: 10.1002/glia.24054
Homogeneity or heterogeneity, the paradox of neurovascular pericytes in the brain
Abstract
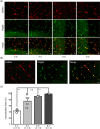
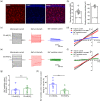
Pericytes are one of the main components of the neurovascular unit. They play a critical role in regulating blood flow, blood-brain barrier permeability, neuroinflammation, and neuronal activity. In the central nervous system (CNS), pericytes are classified into three subtypes, that is, ensheathing, mesh, and thin-strand pericytes, based on their distinct morphologies and region-specific distributions. However, whether these three types of pericytes exhibit heterogeneity or homogeneity with regard to membrane properties has been understudied to date. Here, we combined bulk RNA sequencing analysis with electrophysiological methods to demonstrate that the three subtypes of pericytes share similar electrical membrane properties in the CNS, suggesting a homogenous population of neurovascular pericytes in the brain. Furthermore, we identified an inwardly rectifying potassium channel subtype Kir4.1 functionally expressed in pericytes. Electrophysiological patch clamp recordings indicate that Kir4.1 channel currents in pericytes represent a small portion of the K+ macroscopic currents in physiological conditions. However, a significant augmentation of Kir4.1 currents in pericytes was induced when the extracellular K+ was elevated to pathological levels, suggesting pericytes Kir4.1 channels might play an important role as K+ sensors and contribute to K+ homeostasis in local neurovascular networks in pathology.
Keywords: Kir4.1 ion channels; electrical membrane properties; neurovascular unit; pericyte; potassium sensor.
© 2021 The Authors. GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Bondjers, C., He, L., Takemoto, M., Norlin, J., Asker, N., Hellström, M., … Betsholtz, C. (2006). Microarray analysis of blood microvessels from PDGF‐B and PDGF‐Rbeta mutant mice identifies novel markers for brain pericytes. Federation of American Societies for Experimental Biology Journal, 20(10), 1703–1705. 10.1096/fj.05-4944fje - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

