A Dichotomy in Cross-Coupling Site Selectivity in a Dihalogenated Heteroarene: Influence of Mononuclear Pd, Pd Clusters, and Pd Nanoparticles-the Case for Exploiting Pd Catalyst Speciation
- PMID: 34152135
- PMCID: PMC8297865
- DOI: 10.1021/jacs.1c05294
A Dichotomy in Cross-Coupling Site Selectivity in a Dihalogenated Heteroarene: Influence of Mononuclear Pd, Pd Clusters, and Pd Nanoparticles-the Case for Exploiting Pd Catalyst Speciation
Abstract
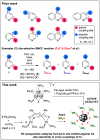
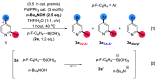
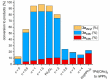
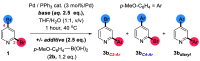
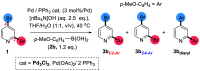
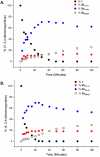
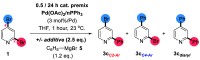
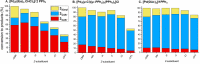
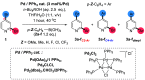
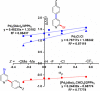
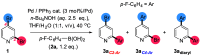
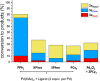
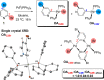
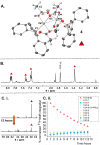
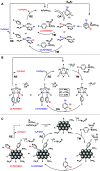
Site-selective dihalogenated heteroarene cross-coupling with organometallic reagents usually occurs at the halogen proximal to the heteroatom, enabled by intrinsic relative electrophilicity, particularly in strongly polarized systems. An archetypical example is the Suzuki-Miyaura cross-coupling (SMCC) of 2,4-dibromopyridine with organoboron species, which typically exhibit C2-arylation site-selectivity using mononuclear Pd (pre)catalysts. Given that Pd speciation, particularly aggregation, is known to lead to the formation of catalytically competent multinuclear Pdn species, the influence of these species on cross-coupling site-selectivity remains largely unknown. Herein, we disclose that multinuclear Pd species, in the form of Pd3-type clusters and nanoparticles, switch arylation site-selectivity from C2 to C4, in 2,4-dibromopyridine cross-couplings with both organoboronic acids (SMCC reactions) and Grignard reagents (Kumada-type reactions). The Pd/ligand ratio and the presence of suitable stabilizing salts were found to be critically important in switching the site-selectivity. More generally, this study provides experimental evidence that aggregated Pd catalyst species not only are catalytically competent but also alter reaction outcomes through changes in product selectivity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

