Enantioselective Iridium-Catalyzed Allylation of Nitroalkanes: Entry to β-Stereogenic α-Quaternary Primary Amines
- PMID: 34152145
- PMCID: PMC8284932
- DOI: 10.1021/jacs.1c05212
Enantioselective Iridium-Catalyzed Allylation of Nitroalkanes: Entry to β-Stereogenic α-Quaternary Primary Amines
Abstract
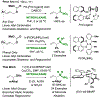
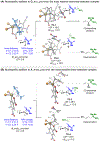
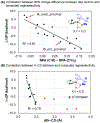
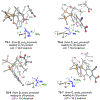
The first systematic study of simple nitronate nucleophiles in iridium-catalyzed allylic alkylation is described. Using a tol-BINAP-modified π-allyliridium C,O-benzoate catalyst, α,α-disubstituted nitronates substitute racemic branched alkyl-substituted allylic acetates, thus providing entry to β-stereogenic α-quaternary primary amines. DFT calculations reveal early transition states that render the reaction less sensitive to steric effects and distinct trans-effects of diastereomeric chiral-at-iridium π-allyl complexes that facilitate formation of congested tertiary-quaternary C-C bonds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Chiral Amines via Enantioselective π-Allyliridium-C,O-Benzoate-Catalyzed Allylic Alkylation: Student Training via Industrial-Academic Collaboration.Acc Chem Res. 2022 Aug 2;55(15):2138-2147. doi: 10.1021/acs.accounts.2c00302. Epub 2022 Jul 13. Acc Chem Res. 2022. PMID: 35830564 Free PMC article.
-
Regio- and Enantioselective Iridium-Catalyzed N-Allylation of Indoles and Related Azoles with Racemic Branched Alkyl-Substituted Allylic Acetates.Angew Chem Int Ed Engl. 2019 Jun 3;58(23):7762-7766. doi: 10.1002/anie.201902799. Epub 2019 May 6. Angew Chem Int Ed Engl. 2019. PMID: 30964961 Free PMC article.
-
Regio- and Enantioselective Iridium-Catalyzed Amination of Alkyl-Substituted Allylic Acetates with Secondary Amines.Org Lett. 2022 Jan 14;24(1):441-445. doi: 10.1021/acs.orglett.1c04135. Epub 2021 Dec 14. Org Lett. 2022. PMID: 34905364 Free PMC article.
-
Enantioselective Formation of Quaternary Centers by Allylic Alkylation with First-Row Transition-Metal Catalysts.Chem Rev. 2021 Apr 14;121(7):4084-4099. doi: 10.1021/acs.chemrev.0c01115. Epub 2021 Feb 11. Chem Rev. 2021. PMID: 33570909 Free PMC article. Review.
-
Enantioselective iridium-catalyzed carbonyl allylation from the alcohol oxidation level via transfer hydrogenation: minimizing pre-activation for synthetic efficiency.Chem Commun (Camb). 2009 Dec 21;(47):7278-87. doi: 10.1039/b917243m. Epub 2009 Oct 16. Chem Commun (Camb). 2009. PMID: 20024203 Free PMC article. Review.
Cited by
-
Kinetic, ESI-CID-MS and Computational Studies of π-Allyliridium C,O-Benzoate-Catalyzed Allylic Amination: Understanding the Effect of Cesium Ion.ACS Catal. 2022 Mar 18;12(6):3660-3668. doi: 10.1021/acscatal.2c00470. Epub 2022 Mar 8. ACS Catal. 2022. PMID: 36092640 Free PMC article.
-
Organocatalytic Michael Addition of Unactivated α-Branched Nitroalkanes to Afford Optically Active Tertiary Nitrocompounds.Org Lett. 2023 Dec 8;25(48):8590-8595. doi: 10.1021/acs.orglett.3c03340. Epub 2023 Nov 27. Org Lett. 2023. PMID: 38009850 Free PMC article.
-
Three-Component cine,ipso-Disubstitution of Nitrocoumarins.Org Lett. 2024 Jan 26;26(3):647-652. doi: 10.1021/acs.orglett.3c03996. Epub 2024 Jan 12. Org Lett. 2024. PMID: 38215699 Free PMC article.
-
Transition-metal free C-N bond formation from alkyl iodides and diazonium salts via halogen-atom transfer.Nat Commun. 2022 Dec 27;13(1):7961. doi: 10.1038/s41467-022-35613-7. Nat Commun. 2022. PMID: 36575172 Free PMC article.
-
Chiral Amines via Enantioselective π-Allyliridium-C,O-Benzoate-Catalyzed Allylic Alkylation: Student Training via Industrial-Academic Collaboration.Acc Chem Res. 2022 Aug 2;55(15):2138-2147. doi: 10.1021/acs.accounts.2c00302. Epub 2022 Jul 13. Acc Chem Res. 2022. PMID: 35830564 Free PMC article.
References
-
- It was estimated in 2014 that chiral amines constitute roughly 40% of new FDA approved small molecule drugs:
- Jarvis LM Chem. Eng. News 2016, 94, 12–17.
-
- For selected reviews on the synthesis of chiral amines, see:
- Enders D; Reinhold U Asymmetric Synthesis of Amines by Nucleophilic 1,2-Addition of Organometallic Reagents to the CN-Double Bond. Tetrahedron: Asymmetry 1997, 8, 1895–1946.
- Bloch R Additions of Organometallic Reagents to C=N Bonds: Reactivity and Selectivity. Chem. Rev 1998, 98, 1407–1438. - PubMed
- Robak MT; Herbage MA; Ellman JA Synthesis and Applications of tert-Butanesulfinamide. Chem. Rev 2010, 110, 3600–3740. - PubMed
- Chiral Amine Synthesis: Methods, Developments and Applications; Nugent TC, Eds.; Wiley-VCH, 2010.
- Nugent TC; El-Shazly M Chiral Amine Synthesis − Recent Developments and Trends for Enamide Reduction, Reductive Amination, and Imine Reduction. Adv. Synth. Catal 2010, 352, 753–819.
- Stereoselective Formation of Amines; Li W, Zhang X, Eds.; Topics in Current Chemistry,; Vol. 343; Springer, 2014. - PubMed
- Mailyan AK; Eickhoff JA; Minakova AS; Gu Z; Lu P; Zakarian A Cutting-Edge and Time-Honored Strategies for Stereoselective Construction of C–N Bonds in Total Synthesis. Chem. Rev 2016, 116, 4441–4557. - PubMed
- Abdine RAA; Hedouin G; Colobert F; Wencel-Delord J Metal-Catalyzed Asymmetric Hydrogenation of C═N Bonds. ACS Catal. 2021, 11, 215–247.
-
- For chiral β-stereogenic amines via catalytic enantioselective hydrogenation of enamines derivatives (including dehydro-β-amino acid derivatives), see:
- Elaridi J; Thaqi A; Prosser A; Jackson WR Robinson, A. J. An Enantioselective Synthesis of β2-Amino Acid Derivatives. Tetrahedron: Asymmetry 2005, 16, 1309–1319.
- Remarchuk T; Babu S; Stults J; Zanotti-Gerosa A; Roseblade S; Yang S; Huang P; Sha C; Wang Y An Efficient Catalytic Asymmetric Synthesis of a β2-Amino Acid on Multikilogram Scale. Org. Process Res. Dev 2014, 18, 135–141.
- Saito N; Abdullah I; Hayashi K; Hamada K; Koyama M; Sato Y Enantioselective Synthesis of β-Amino Acid Derivatives via Nickel-Promoted Regioselective Carboxylation of Ynamides and Rhodium-Catalyzed Asymmetric Hydrogenation. Org. Biomol. Chem 2016, 14, 10080–10089. - PubMed
- Han C; Savage S; Al-Sayah M; Yajima H; Remarchuk T; Reents R; Wirz B; Iding H; Bachmann S; Fantasia SM; Scalone M; Hell A; Hidber P; Gosselin F Asymmetric Synthesis of Akt Kinase Inhibitor Ipatasertib. Org. Lett 2017, 19, 4806–4809. - PubMed
- Zhang J; Liu C; Wang X; Chen J; Zhang Z; Zhang W Rhodium-Catalyzed Asymmetric Hydrogenation of β-Branched Enamides for the Synthesis of β-Stereogenic Amines. Chem. Commun 2018, 54, 6024–6027. - PubMed
-
- For chiral β-stereogenic amines via catalytic enantioselective hydrogenation of allylic amines or amides to form acyclic chiral β-stereogenic amines, see:
- Fahrang R; Sinou D Asymmetric Reduction of Allylamines and their Derivatives with Rhodium Complexes. Bull. Soc. Chim. Belg 1989, 98, 387–393.
- Wang C-J; Sun X; Zhang X Enantioselective Hydrogenation of Allylphthalimides: An Efficient Method for the Synthesis of β-Methyl Chiral Amines. Angew. Chem., Int. Ed 2005, 44, 4933–4935. - PubMed
- Steinhuebel DP; Krska SW; Alorati A; Baxter JM; Belyk K; Bishop B; Palucki M; Sun Y; Davies IW Asymmetric Hydrogenation of Protected Allylic Amines. Org. Lett 2010, 12, 4201–4203. - PubMed
- Ma S; Grinberg N; Haddad N; Rodriguez S; Busacca CA; Fandrick K; Lee H; Song JJ; Yee N; Krishnamurthy D; Senanayake CH; Wang J; Trenck J; Mendonsa S; Claise PR; Gilman RJ; Evers TH Org. Process Res. Dev 2013, 17, 806–810.
- Cabre A; Verdaguer X; Riera A Enantioselective Synthesis of β-Methyl Amines via Iridium-Catalyzed Asymmetric Hydrogenation of N-Sulfonyl Allyl Amines. Adv. Synth. Catal 2019, 361, 4196–4200.
-
- For chiral β-stereogenic amines via catalytic enantioselective hydrogenation of nitrolefins, see:
- Li S; Huang K; Cao B; Zhang J; Wu W; Zhang X Highly Enantioselective Hydrogenation of β,β-Disubstituted Nitroalkanes. Angew. Chem., Int. Ed 2012, 51, 8573–8576. - PubMed
- Li S; Huang K; Zhang J; Wu W; Zhang X Rh-Catalyzed Highly Enantioselective Hydrogenation of Nitroalkenes under Basic Conditions. Chem. Eur. J 2013, 19, 10840–10844. - PubMed
- Zhao Q; Li S; Huang K; Wang R; Zhang X A Novel Chiral Bisphosphine-Thiourea Ligand for Asymmetric Hydrogenation of β,β-Disubstituted Nitroalkenes. Org. Lett 2013, 15, 4014–4017. - PubMed
- Liu M, Kong D, Li M, Zi G and Hou G, Iridium-Catalyzed Enantioselective Hydrogenation of β,β-Disubstituted Nitroalkenes. Adv. Synth. Catal 2015, 357, 3875–3879.
- Li S; Xiao T; Li D; Zhang X First Iridium-Catalyzed Highly Enantioselective Hydrogenation of β-Nitroacrylates. Org. Lett 2015, 17, 3782–3785. - PubMed
- Yu Y-B; Cheng L; Li Y-P; Fu Y; Zhu S-F; Zhou Q-L Enantioselective Iridium-Catalyzed Hydrogenation of β,β-Disubstituted Nitroalkenes. Chem. Commun 2016, 52, 4812–4815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

