Efficacy of Smartphone Active and Passive Virtual Reality Distraction vs Standard Care on Burn Pain Among Pediatric Patients: A Randomized Clinical Trial
- PMID: 34152420
- PMCID: PMC8218073
- DOI: 10.1001/jamanetworkopen.2021.12082
Efficacy of Smartphone Active and Passive Virtual Reality Distraction vs Standard Care on Burn Pain Among Pediatric Patients: A Randomized Clinical Trial
Abstract
Importance: It is unknown whether smartphone-based virtual reality (VR) games are effective in reducing pain among pediatric patients in real-world burn clinics.
Objective: To evaluate the efficacy of a smartphone VR game on dressing pain among pediatric patients with burns.
Design, setting, and participants: This randomized clinical trial included children aged 6 to 17 years who seen in the outpatient clinic of a large American Burn Association-verified pediatric burn center and level I pediatric trauma center between December 30, 2016, and January 23, 2019. Speaking English as their primary language was an inclusion criterion. Intention-to-treat data analyses were conducted from December 2019 to March 2020.
Interventions: Active VR participants played a VR game; passive VR participants were immersed in the same VR environment without interactions. Both groups were compared with a standard care group. One researcher administered VR and observed pain while another researcher administered a posttrial survey that measured the child's perceived pain and VR experience. Nurses were asked to report the clinical utility.
Main outcomes and measures: Patients self-reported pain using a visual analog scale (VAS; range, 0-100). A researcher observed patient pain based on the Face, Legs, Activity, Cry, and Consolability-Revised (FLACC-R) scale. Nurses were asked to report VR helpfulness (range, 0-100; higher scores indicate more helpful) and ease of use (range, 0-100; higher scores indicate easier to use).
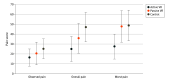
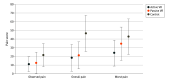
Results: A total of 90 children (45 [50%] girls, mean age, 11.3 years [95% CI, 10.6-12.0 years]; 51 [57%] White children) participated. Most children had second-degree burns (81 [90%]). Participants in the active VR group had significantly lower reported overall pain (VAS score, 24.9 [95% CI, 12.2-37.6]) compared with participants in the standard care control group (VAS score, 47.1 [95% CI, 32.1-62.2]; P = .02). The active VR group also had a lower worst pain score (VAS score, 27.4 [95% CI, 14.7-40.1]) than both the passive VR group (VAS score, 47.9 [95% CI, 31.8-63.9]; P = .04) and the standard care group (VAS score, 48.8 [95% CI, 31.1-64.4]; P = .03). Simulator sickness scores (range, 0-60; lower scores indicate less sickness) were similar for active VR (19.3 [95% CI, 17.5-21.1]) and passive VR groups (19.5 [95% CI, 17.6-21.5]). Nurses also reported that the VR games could be easily implemented in clinics (helpfulness, active VR: 84.2; 95% CI, 74.5-93.8; passive VR: 76.9; 95% CI, 65.2-88.7; ease of use, active VR: 94.8, 95% CI, 91.8-97.8; passive VR: 96.0, 95% CI, 92.9-99.1).
Conclusions and relevance: In this study, a smartphone VR game was effective in reducing patient self-reported pain during burn dressing changes, suggesting that VR may be an effective method for managing pediatric burn pain.
Trial registration: ClinicalTrials.gov Identifier: NCT04544631.
Conflict of interest statement
Figures