A Case Study in Catalyst Generality: Simultaneous, Highly-Enantioselective Brønsted- and Lewis-Acid Mechanisms in Hydrogen-Bond-Donor Catalyzed Oxetane Openings
- PMID: 34152759
- PMCID: PMC8564877
- DOI: 10.1021/jacs.1c03992
A Case Study in Catalyst Generality: Simultaneous, Highly-Enantioselective Brønsted- and Lewis-Acid Mechanisms in Hydrogen-Bond-Donor Catalyzed Oxetane Openings
Abstract
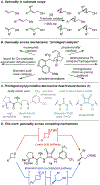
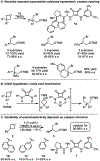
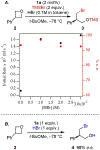
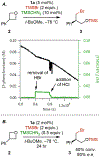
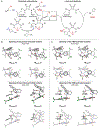
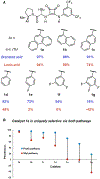
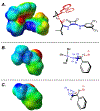
Generality in asymmetric catalysis can be manifested in dramatic and valuable ways, such as high enantioselectivity across a wide assortment of substrates in a given reaction (broad substrate scope) or as applicability of a given chiral framework across a variety of mechanistically distinct reactions (privileged catalysts). Reactions and catalysts that display such generality hold special utility, because they can be applied broadly and sometimes even predictably in new applications. Despite the great value of such systems, the factors that underlie generality are not well understood. Here, we report a detailed investigation of an asymmetric hydrogen-bond-donor catalyzed oxetane opening with TMSBr that is shown to possess unexpected mechanistic generality. Careful analysis of the role of adventitious protic impurities revealed the participation of competing pathways involving addition of either TMSBr or HBr in the enantiodetermining, ring-opening event. The optimal catalyst induces high enantioselectivity in both pathways, thereby achieving precise stereocontrol in fundamentally different mechanisms under the same conditions and with the same chiral framework. The basis for that generality is analyzed using a combination of experimental and computational methods, which indicate that proximally localized catalyst components cooperatively stabilize and precisely orient dipolar enantiodetermining transition states in both pathways. Generality across different mechanisms is rarely considered in catalyst discovery efforts, but we suggest that it may play a role in the identification of so-called privileged catalysts.
Figures
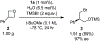

References
-
- Knowles WS Asymmetric Hydrogenations (Nobel Lecture). Andrew. Chem., Int. Ed 2002, 41, 1998–2007. - PubMed
- For an authoritative historical perspective, see: Kagan HB in Comprehensive Asymmetric Catalysis. Jacobsen EN; Pfaltz A; Yamamoto H, Eds., Springer: New York, 1999; Vol. 1, Ch. 1, pp. 9–30.
-
- Some notable examples: Gao Y; Klunder JM; Hanson RM; Masamune H; Ko SY; Sharpless KB Catalytic asymmetric epoxidation and kinetic resolution: modified procedures including in situ derivatization. J. Am. Chem. Soc 1987, 109, 5765–5780.
- Kolb HC; VanNieuwenhze MS; Sharpless KB Catalytic Asymmetric Dihydroxylation. Chem. Rev 1994, 94, 2483–2547.
- Corey EJ; Helal CJ Reduction of Carbonyl Compounds with Chiral Oxazaborolidine Catalysts: A New Paradigm for Enantioselective Catalysis and a Powerful New Synthetic Method. Angew. Chem., Int. Ed. 1998, 37, 1986–2012. - PubMed
- Noyori R; Takeshi O Asymmetric Catalysis by Architectural and Functional Molecular Engineering: Practical Chemo- and Stereoselective Hydrogenation of Ketones Angew. Chem., Int. Ed 2001, 40, 40–73. - PubMed
- Schaus SE; Brandes BD; Larrow JF; Tokunaga M; Hansen KB; Gould AE; Furrow ME; Jacobsen EN Highly Selective Hydrolytic Kinetic Resolution of Terminal Epoxides Catalyzed by Chiral (salen)Cobalt(III)-Complexes. Practical Synthesis of Enantioenriched Terminal Epoxides and 1,2-Diols. J. Am. Chem. Soc 2002, 124, 1307–1315 - PubMed
-
- Finn MG; Sharpless KB Mechanism of asymmetric epoxidation. 2. Catalyst structure. J. Am. Chem. Soc 1991, 113, 113–126.
- Ford DD; Nielsen LPC; Zuend SJ; Musgrave CB; Jacobsen EN Mechanistic Basis for High Stereoselectivity and Broad Substrate Scope in the (salen)Co(III)-Catalyzed Hydrolytic Kinetic Resolution. J. Am. Chem. Soc 2013, 135, 15595–15608. - PMC - PubMed
-
- Yoon TP; Jacobsen EN Privileged chiral catalysts. Science 2003, 299, 1691–1693. - PubMed
- Intriguingly, a similar phenomenon has been noted in enzymatic catalysis, wherein particular protein folds appear to be particularly well suited for catalyzing mechanistically-distinct reactions: Anantharaman V; Aravind L; Koonin EV Emergence of diverse biochemical activities in evolutionarily conserved structural scaffolds of proteins. Curr. Op. Chem. Biol 2003, 7, 12–20. - PubMed
-
- In the arena of asymmetric hydrogenation in particular, it is common to rely on known chiral ligand scaffolds for the identification of new applications. For a highly noteworthy, but representative example, see: Hansen KB; Hsiao Y; River N; Clausen A; Kubryk M; Krska S; Rosner T; Simmons B; Balsells J; Ikemoto N; Sun Y; Spindler F; Malan C; Grabowski EJJ; Armstrong JD III Highly Efficient Asymmetric Synthesis of Sitagliptin. J. Am. Chem. Soc 2009, 131, 8798–8804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

