Novel Nonnucleoside Inhibitors of Zika Virus Polymerase Identified through the Screening of an Open Library of Antikinetoplastid Compounds
- PMID: 34152807
- PMCID: PMC8370225
- DOI: 10.1128/AAC.00894-21
Novel Nonnucleoside Inhibitors of Zika Virus Polymerase Identified through the Screening of an Open Library of Antikinetoplastid Compounds
Abstract
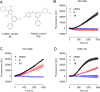
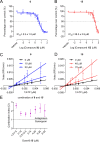
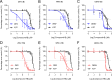
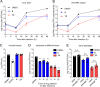
Zika virus (ZIKV) is a mosquito-borne pathogen responsible for neurological disorders (Guillain-Barré syndrome) and congenital malformations (microcephaly). Its ability to cause explosive epidemics, such as that of 2015 to 2016, urges the identification of effective antiviral drugs. Viral polymerase inhibitors constitute one of the most successful fields in antiviral research. Accordingly, the RNA-dependent RNA polymerase activity of flavivirus nonstructural protein 5 (NS5) provides a unique target for the development of direct antivirals with high specificity and low toxicity. Here, we describe the discovery and characterization of two novel nonnucleoside inhibitors of ZIKV polymerase. These inhibitors, TCMDC-143406 (compound 6) and TCMDC-143215 (compound 15) were identified through the screening of an open-resource library of antikinetoplastid compounds using a fluorescence-based polymerization assay based on ZIKV NS5. The two compounds inhibited ZIKV NS5 polymerase activity in vitro and ZIKV multiplication in cell culture (half-maximal effective concentrations [EC50] values of 0.5 and 2.6 μM for compounds 6 and 15, respectively). Both compounds also inhibited the replication of other pathogenic flaviviruses, namely, West Nile virus (WNV; EC50 values of 4.3 and 4.6 μM for compounds 6 and 15, respectively) and dengue virus 2 (DENV-2; EC50 values of 3.4 and 9.6 μM for compounds 6 and 15, respectively). Enzymatic assays confirmed that the polymerase inhibition was produced by a noncompetitive mechanism. Combinatorial assays revealed an antagonistic effect between both compounds, suggesting that they would bind to the same region of ZIKV polymerase. The nonnucleoside inhibitors of ZIKV polymerase here described could constitute promising lead compounds for the development of anti-ZIKV therapies and, eventually, broad-spectrum antiflavivirus drugs.
Keywords: RNA polymerases; West Nile virus; Zika virus; allosteric; antiviral; antiviral agents; dengue virus; nonnucleoside inhibitor; polymerase.
Figures
Similar articles
-
Non-nucleoside Inhibitors of Zika Virus RNA-Dependent RNA Polymerase.J Virol. 2020 Oct 14;94(21):e00794-20. doi: 10.1128/JVI.00794-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32796069 Free PMC article.
-
Small-Molecule Inhibitor of Flaviviral NS3-NS5 Interaction with Broad-Spectrum Activity and Efficacy In Vivo.mBio. 2023 Feb 28;14(1):e0309722. doi: 10.1128/mbio.03097-22. Epub 2023 Jan 9. mBio. 2023. PMID: 36622141 Free PMC article.
-
Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay.Antiviral Res. 2017 Feb;138:47-56. doi: 10.1016/j.antiviral.2016.11.018. Epub 2016 Dec 3. Antiviral Res. 2017. PMID: 27919709
-
New avenues for therapeutic discovery against West Nile virus.Expert Opin Drug Discov. 2020 Mar;15(3):333-348. doi: 10.1080/17460441.2020.1714586. Epub 2020 Feb 4. Expert Opin Drug Discov. 2020. PMID: 32017639 Review.
-
Advances in Computational Methods to Discover New NS2B-NS3 Inhibitors Useful Against Dengue and Zika Viruses.Curr Top Med Chem. 2022;22(29):2435-2462. doi: 10.2174/1568026623666221122121330. Curr Top Med Chem. 2022. PMID: 36415099 Review.
Cited by
-
Azelnidipine Exhibits In Vitro and In Vivo Antiviral Effects against Flavivirus Infections by Targeting the Viral RdRp.Viruses. 2022 Jun 5;14(6):1228. doi: 10.3390/v14061228. Viruses. 2022. PMID: 35746699 Free PMC article.
-
Advances in antiviral strategies targeting mosquito-borne viruses: cellular, viral, and immune-related approaches.Virol J. 2025 Feb 4;22(1):26. doi: 10.1186/s12985-025-02622-z. Virol J. 2025. PMID: 39905499 Free PMC article. Review.
-
A review on structural genomics approach applied for drug discovery against three vector-borne viral diseases: Dengue, Chikungunya and Zika.Virus Genes. 2022 Jun;58(3):151-171. doi: 10.1007/s11262-022-01898-5. Epub 2022 Apr 8. Virus Genes. 2022. PMID: 35394596 Review.
-
Allosteric Inhibition of Neutral Sphingomyelinase 2 (nSMase2) by DPTIP: From Antiflaviviral Activity to Deciphering Its Binding Site through In Silico Studies and Experimental Validation.Int J Mol Sci. 2022 Nov 11;23(22):13935. doi: 10.3390/ijms232213935. Int J Mol Sci. 2022. PMID: 36430407 Free PMC article.
-
Novel and repurposed antiviral molecules for arbovirus infections with epidemic Potential: A systematic review.New Microbes New Infect. 2025 Jul 10;66:101614. doi: 10.1016/j.nmni.2025.101614. eCollection 2025 Aug. New Microbes New Infect. 2025. PMID: 40776987 Free PMC article. Review.
References
-
- Saiz JC, Martin-Acebes MA, Bueno-Mari R, Salomon OD, Villamil-Jimenez LC, Heukelbach J, Alencar CH, Armstrong PK, Ortiga-Carvalho TM, Mendez-Otero R, Rosado-de-Castro PH, Pimentel-Coelho PM. 2017. Zika virus: what have we learnt since the start of the recent epidemic? Front Microbiol 8:1554. 10.3389/fmicb.2017.01554. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

