Amaryllidaceae Alkaloid Cherylline Inhibits the Replication of Dengue and Zika Viruses
- PMID: 34152811
- PMCID: PMC8370201
- DOI: 10.1128/AAC.00398-21
Amaryllidaceae Alkaloid Cherylline Inhibits the Replication of Dengue and Zika Viruses
Abstract
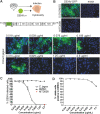
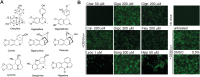
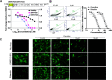
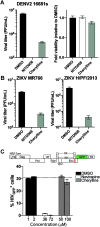
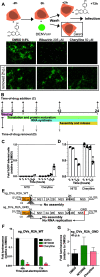
Dengue fever, caused by dengue virus (DENV), is the most prevalent arthropod-borne viral disease and is endemic in many tropical and subtropical parts of the world, with an increasing incidence in temperate regions. The closely related flavivirus Zika virus (ZIKV) can be transmitted vertically in utero and causes congenital Zika syndrome and other birth defects. In adults, ZIKV is associated with Guillain-Barré syndrome. There are no approved antiviral therapies against either virus. Effective antiviral compounds are urgently needed. Amaryllidaceae alkaloids (AAs) are a specific class of nitrogen-containing compounds produced by plants of the Amaryllidaceae family with numerous biological activities. Recently, the AA lycorine was shown to present strong antiflaviviral properties. Previously, we demonstrated that Crinum jagus contained lycorine and several alkaloids of the cherylline, crinine, and galanthamine types with unknown antiviral potential. In this study, we explored their biological activities. We show that C. jagus crude alkaloid extract inhibited DENV infection. Among the purified AAs, cherylline efficiently inhibited both DENV (50% effective concentration [EC50], 8.8 μM) and ZIKV replication (EC50, 20.3 μM) but had no effect on HIV-1 infection. Time-of-drug-addition and -removal experiments identified a postentry step as the one targeted by cherylline. Consistently, using subgenomic replicons and replication-defective genomes, we demonstrate that cherylline specifically hinders the viral RNA synthesis step but not viral translation. In conclusion, AAs are an underestimated source of antiflavivirus compounds, including the effective inhibitor cherylline, which could be optimized for new therapeutic approaches.
Keywords: Amaryllidaceae; Zika virus; alkaloids; antivirals; cherylline; dengue virus; flavivirus; lycorine.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

