Effects of COVID-19 Vaccination Timing and Risk Prioritization on Mortality Rates, United States
- PMID: 34152963
- PMCID: PMC8237869
- DOI: 10.3201/eid2707.210118
Effects of COVID-19 Vaccination Timing and Risk Prioritization on Mortality Rates, United States
Abstract
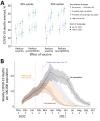
During rollout of coronavirus disease vaccination, policymakers have faced critical trade-offs. Using a mathematical model of transmission, we found that timing of vaccination rollout would be expected to have a substantially greater effect on mortality rate than risk-based prioritization and uptake and that prioritizing first doses over second doses may be lifesaving.
Keywords: COVID-19; SARS-CoV-2; Texas; United States; coronavirus disease; epidemiology; mathematical model; mortality; pandemics; respiratory infections; severe acute respiratory syndrome coronavirus 2; vaccines; viruses; zoonoses.
Figures
Similar articles
-
Considerations for Establishing Successful Coronavirus Disease Vaccination Programs in Africa.Emerg Infect Dis. 2021 Aug;27(8):2009-2016. doi: 10.3201/eid2708.203870. Epub 2021 Jun 17. Emerg Infect Dis. 2021. PMID: 34138694 Free PMC article. Review.
-
Disparities in First Dose COVID-19 Vaccination Coverage among Children 5-11 Years of Age, United States.Emerg Infect Dis. 2022 May;28(5):986-989. doi: 10.3201/eid2805.220166. Epub 2022 Feb 28. Emerg Infect Dis. 2022. PMID: 35226801 Free PMC article.
-
Multisystem Inflammatory Syndrome after SARS-CoV-2 Infection and COVID-19 Vaccination.Emerg Infect Dis. 2021 Jul;27(7):1944-1948. doi: 10.3201/eid2707.210594. Epub 2021 May 25. Emerg Infect Dis. 2021. PMID: 34034858 Free PMC article.
-
COVID-19 Vaccination Coverage, Intent, Knowledge, Attitudes, and Beliefs among Essential Workers, United States.Emerg Infect Dis. 2021 Nov;27(11):2908-2913. doi: 10.3201/eid2711.211557. Epub 2021 Sep 29. Emerg Infect Dis. 2021. PMID: 34586060 Free PMC article.
-
Human and novel coronavirus infections in children: a review.Paediatr Int Child Health. 2021 Feb;41(1):36-55. doi: 10.1080/20469047.2020.1781356. Epub 2020 Jun 25. Paediatr Int Child Health. 2021. PMID: 32584199 Review.
Cited by
-
Phylodynamic of SARS-CoV-2 during the second wave of COVID-19 in Peru.Nat Commun. 2023 Jun 15;14(1):3557. doi: 10.1038/s41467-023-39216-8. Nat Commun. 2023. PMID: 37322028 Free PMC article.
-
Studying Disease Reinfection Rates, Vaccine Efficacy, and the Timing of Vaccine Rollout in the Context of Infectious Diseases: A COVID-19 Case Study.Int J Environ Res Public Health. 2025 May 3;22(5):731. doi: 10.3390/ijerph22050731. Int J Environ Res Public Health. 2025. PMID: 40427847 Free PMC article.
-
Modeling comparative cost-effectiveness of SARS-CoV-2 vaccine dose fractionation in India.Nat Med. 2022 May;28(5):934-938. doi: 10.1038/s41591-022-01736-z. Epub 2022 Feb 24. Nat Med. 2022. PMID: 35210596 Free PMC article.
-
Impact of Anxiety on Readiness for COVID-19 Vaccination among Polish Nursing Undergraduate Students: Nationwide Cross-Sectional Study.Vaccines (Basel). 2021 Nov 24;9(12):1385. doi: 10.3390/vaccines9121385. Vaccines (Basel). 2021. PMID: 34960130 Free PMC article.
-
Optimizing COVID-19 vaccination programs during vaccine shortages.Infect Dis Model. 2022 Mar;7(1):286-298. doi: 10.1016/j.idm.2022.02.002. Epub 2022 Feb 25. Infect Dis Model. 2022. PMID: 35233475 Free PMC article.
References
-
- Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Moderna COVID-19 vaccine. December 17, 2020. [cited 2021 May 7]. https://www.fda.gov/media/144434/download
-
- Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Pfizer BioNTech COVID-19 vaccine. December 10, 2020. [cited 2021 May 7]. https://www.fda.gov/media/144245/download
-
- US Food and Drug Administration. COVID-19 vaccines. [cited 2021 Feb 17]. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise...
-
- Dooling K, McClung N, Chamberland M, Marin M, Wallace M, Bell BP, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857–9. 10.15585/mmwr.mm6949e1 - DOI - PMC - PubMed
-
- US Centers for Disease Control and Prevention. Vaccines for COVID-19. 2021. [cited 2021 Feb 15]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

