Cytomegalovirus mediates expansion of IL-15-responsive innate-memory cells with SIV killing function
- PMID: 34153005
- PMCID: PMC8321572
- DOI: 10.1172/JCI148542
Cytomegalovirus mediates expansion of IL-15-responsive innate-memory cells with SIV killing function
Abstract
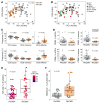
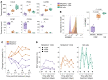
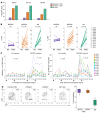
Interindividual immune variability is driven predominantly by environmental factors, including exposure to chronic infectious agents such as cytomegalovirus (CMV). We investigated the effects of rhesus CMV (RhCMV) on composition and function of the immune system in young macaques. Within months of infection, RhCMV was associated with impressive changes in antigen presenting cells, T cells, and NK cells-and marked expansion of innate-memory CD8+ T cells. These cells express high levels of NKG2A/C and the IL-2 and IL-15 receptor beta chain, CD122. IL-15 was sufficient to drive differentiation of the cells in vitro and in vivo. Expanded NKG2A/C+CD122+CD8+ T cells in RhCMV-infected macaques, but not their NKG2-negative counterparts, were endowed with cytotoxicity against class I-deficient K562 targets and prompt IFN-γ production in response to stimulation with IL-12 and IL-18. Because RhCMV clone 68-1 forms the viral backbone of RhCMV-vectored SIV vaccines, we also investigated immune changes following administration of RhCMV 68-1-vectored SIV vaccines. These vaccines led to impressive expansion of NKG2A/C+CD8+ T cells with capacity to inhibit SIV replication ex vivo. Thus, CMV infection and CMV-vectored vaccination drive expansion of functional innate-like CD8 cells via host IL-15 production, suggesting that innate-memory expansion could be achieved by other vaccine platforms expressing IL-15.
Keywords: Cellular immune response; Immunology; Infectious disease; Innate immunity; T cells.
Conflict of interest statement
Figures



Similar articles
-
Interleukin-15 response signature predicts RhCMV/SIV vaccine efficacy.PLoS Pathog. 2021 Jul 6;17(7):e1009278. doi: 10.1371/journal.ppat.1009278. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34228762 Free PMC article.
-
Cytomegaloviral determinants of CD8+ T cell programming and RhCMV/SIV vaccine efficacy.Sci Immunol. 2021 Mar 25;6(57):eabg5413. doi: 10.1126/sciimmunol.abg5413. Sci Immunol. 2021. PMID: 33766849 Free PMC article.
-
Rhesus Cytomegalovirus-Specific CD8+ Cytotoxic T Lymphocytes Do Not Become Functionally Exhausted in Chronic SIVmac239 Infection.Front Immunol. 2020 Aug 12;11:1960. doi: 10.3389/fimmu.2020.01960. eCollection 2020. Front Immunol. 2020. PMID: 32922404 Free PMC article.
-
NK-like CD8 T cell: one potential evolutionary continuum between adaptive memory and innate immunity.Clin Exp Immunol. 2024 Jul 12;217(2):136-150. doi: 10.1093/cei/uxae038. Clin Exp Immunol. 2024. PMID: 38651831 Free PMC article. Review.
-
Extrinsic and intrinsic drivers of natural killer cell clonality.Immunol Rev. 2024 May;323(1):80-106. doi: 10.1111/imr.13324. Epub 2024 Mar 20. Immunol Rev. 2024. PMID: 38506411 Review.
Cited by
-
Exploring HIV Vaccine Progress in the Pre-Clinical and Clinical Setting: From History to Future Prospects.Viruses. 2024 Feb 27;16(3):368. doi: 10.3390/v16030368. Viruses. 2024. PMID: 38543734 Free PMC article. Review.
-
HIV T-cell immunogen design and delivery.Curr Opin HIV AIDS. 2022 Nov 1;17(6):333-337. doi: 10.1097/COH.0000000000000765. Epub 2022 Sep 19. Curr Opin HIV AIDS. 2022. PMID: 36165078 Free PMC article. Review.
-
Functional genomic analysis of the 68-1 RhCMV-Mycobacteria tuberculosis vaccine reveals an IL-15 response signature that is conserved with vector attenuation.Front Immunol. 2024 Oct 15;15:1460344. doi: 10.3389/fimmu.2024.1460344. eCollection 2024. Front Immunol. 2024. PMID: 39474415 Free PMC article.
-
Revisiting the role of human memory CD8+ T cells in immune surveillance.Cell Mol Immunol. 2022 Nov;19(11):1319-1321. doi: 10.1038/s41423-022-00900-2. Epub 2022 Aug 3. Cell Mol Immunol. 2022. PMID: 35922545 Free PMC article. No abstract available.
-
Prospects for therapeutic T-cell vaccine strategies for HIV cure.Curr Opin HIV AIDS. 2025 Sep 1;20(5):463-471. doi: 10.1097/COH.0000000000000965. Epub 2025 Jul 9. Curr Opin HIV AIDS. 2025. PMID: 40638102 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

