Estrogens in polycystic liver disease: A target for future therapies?
- PMID: 34153174
- PMCID: PMC8456902
- DOI: 10.1111/liv.14986
Estrogens in polycystic liver disease: A target for future therapies?
Abstract
Background and aims: Patients suffering from polycystic liver disease (PLD) can develop large liver volumes, leading to physical and psychological complaints, reducing quality of life. There is an unmet need for new therapies in these patients. Estrogen seems to be a promising target for new therapies. In this review, we summarize the available experimental and epidemiological evidence to unravel the role of estrogens and other female hormones in PLD, to answer clinical questions and identify new targets for therapy.
Methods: We identified all experimental and epidemiologial studies concerning estrogens or other female hormones and PLD, to answer pre-defined clinial questions.
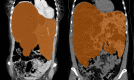
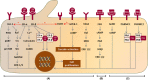
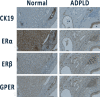
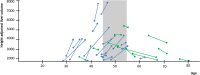
Results: Female sex is the most important risk factor for the presence and severity of disease; estrogen supplementation enhances liver growth and after menopause, liver growth decreases. Experimental studies show the presence of the estrogen receptors alfa and beta on cystic cholangiocytes, and increased in vitro growth after administration of estrogen.
Conclusions: Based on the available evidence, female PLD patients should be discouraged from taking estrogen-containing contraceptives or hormone replacement therapy. Since liver growth rates decline after menopause, treatment decisions should be based on measured liver growth in postmenopausal women. Finally, blockage of estrogen receptors or estrogen production is a promising target for new therapies.
Keywords: ADPKD; GnRH analogues; estrogen; polycystic liver disease; progesterone; tamoxifen.
© 2021 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors received unrestricted grants from the Dutch Government (ZonMW grant 10140261910001) and Abbvie. The organization had any role in neither the conception nor in writing of this manuscript. Dr Gansevoort received grant support and fees for serving on advisory boards and steering committees from Galapagos, IPSEN, Otsuka Pharmaceuticals and Sanofi‐Genzyme. In addition, Dr Gansevoort holds the Orphan Medicinal Product Designation status at the European Medicines Agency for lanreotide as treatment for kidney function decline in ADPKD (EMA/OD/027/15). Dr Drenth has received grant support and fees for serving on advisory boards and consultancy from IPSEN and Novartis. All money is paid to their institutions. No other potential conflict of interest relevant to this article was reported.
Figures

References
-
- Van Aerts RMM, Van De Laarschot LFM, Banales JM, Drenth JPH. Clinical management of polycystic liver disease. J Hepatol. 2018;68:827‐837. - PubMed
-
- Van Keimpema L, De Koning DB, Van Hoek B, et al. Patients with isolated polycystic liver disease referred to liver centres: clinical characterization of 137 cases. Liver Int. 2011;31:92‐98. - PubMed
-
- van Aerts RMM , Kievit W, D’Agnolo HMA, et al. Lanreotide reduces liver growth in patients with autosomal dominant polycystic liver and kidney disease. Gastroenterology. 2019;157:481‐491. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

