An Overview of Microcrystal Electron Diffraction (MicroED)
- PMID: 34153215
- PMCID: PMC9974886
- DOI: 10.1146/annurev-biochem-081720-020121
An Overview of Microcrystal Electron Diffraction (MicroED)
Abstract
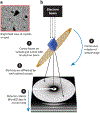
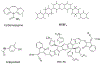
The bedrock of drug discovery and a key tool for understanding cellular function and drug mechanisms of action is the structure determination of chemical compounds, peptides, and proteins. The development of new structure characterization tools, particularly those that fill critical gaps in existing methods, presents important steps forward for structural biology and drug discovery. The emergence of microcrystal electron diffraction (MicroED) expands the application of cryo-electron microscopy to include samples ranging from small molecules and membrane proteins to even large protein complexes using crystals that are one-billionth the size of those required for X-ray crystallography. This review outlines the conception, achievements, and exciting future trajectories for MicroED, an important addition to the existing biophysical toolkit.
Keywords: MicroED; cryo-EM; cryo–electron microscopy; crystallography; microcrystal electron diffraction; proteins; structures.
Figures


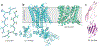
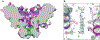


References
-
- Dubochet J, Booyl FP, Freeman R, Jones AV, Walter CA. 1981. Low temperature electron microscopy. Ann. Rev. Biophys 10:133–49 - PubMed
-
- Taylor KA, Glaeser RM. 1974. Electron diffraction of frozen, hydrated protein crystals. Science 186:1036–37 - PubMed
-
- Wang L, Sigworth FJ. 2006. Cryo-EM and single particles. Physiology 21:13–18 - PubMed

