Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial
- PMID: 34153264
- PMCID: PMC8213361
- DOI: 10.1016/S2352-3018(21)00103-X
Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial
Abstract
Background: Data on vaccine immunogenicity against SARS-CoV-2 are needed for the 40 million people globally living with HIV who might have less functional immunity and more associated comorbidities than the general population. We aimed to explore safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine in people with HIV.
Methods: In this single-arm open-label vaccination substudy within the protocol of the larger phase 2/3 trial COV002, adults aged 18-55 years with HIV were enrolled at two HIV clinics in London, UK. Eligible participants were required to be on antiretroviral therapy (ART), with undetectable plasma HIV viral loads (<50 copies per mL), and CD4 counts of more than 350 cells per μL. A prime-boost regimen of ChAdOx1 nCoV-19, with two doses was given 4-6 weeks apart. The primary outcomes for this substudy were safety and reactogenicity of the vaccine, as determined by serious adverse events and solicited local and systemic reactions. Humoral responses were measured by anti-spike IgG ELISA and antibody-mediated live virus neutralisation. Cell-mediated immune responses were measured by ex-vivo IFN-γ enzyme-linked immunospot assay (ELISpot) and T-cell proliferation. All outcomes were compared with an HIV-uninfected group from the main COV002 study within the same age group and dosing strategy and are reported until day 56 after prime vaccination. Outcomes were analysed in all participants who received both doses and with available samples. The COV002 study is registered with ClinicalTrials.gov, NCT04400838, and is ongoing.
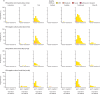
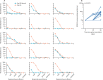
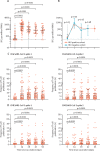
Findings: Between Nov 5 and Nov 24, 2020, 54 participants with HIV (all male, median age 42·5 years [IQR 37·2-49·8]) were enrolled and received two doses of ChAdOx1 nCoV-19. Median CD4 count at enrolment was 694·0 cells per μL (IQR 573·5-859·5). No serious adverse events occurred. Local and systemic reactions occurring during the first 7 days after prime vaccination included pain at the injection site (26 [49%] of 53 participants with available data), fatigue (25 [47%]), headache (25 [47%]), malaise (18 [34%]), chills (12 [23%]), muscle ache (19 [36%]), joint pain (five [9%]), and nausea (four [8%]), the frequencies of which were similar to the HIV-negative participants. Anti-spike IgG responses by ELISA peaked at day 42 (median 1440 ELISA units [EUs; IQR 704-2728]; n=50) and were sustained until day 56 (median 941 EUs [531-1445]; n=49). We found no correlation between the magnitude of the anti-spike IgG response at day 56 and CD4 cell count (p=0·93) or age (p=0·48). ELISpot and T-cell proliferative responses peaked at day 14 and 28 after prime dose and were sustained to day 56. Compared with participants without HIV, we found no difference in magnitude or persistence of SARS-CoV-2 spike-specific humoral or cellular responses (p>0·05 for all analyses).
Interpretation: In this study of people with HIV, ChAdOx1 nCoV-19 was safe and immunogenic, supporting vaccination for those well controlled on ART.
Funding: UK Research and Innovation, National Institutes for Health Research (NIHR), Coalition for Epidemic Preparedness Innovations, NIHR Oxford Biomedical Research Centre, Thames Valley and South Midland's NIHR Clinical Research Network, and AstraZeneca.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19 (AZD1222). SCG is cofounder of Vaccitech (a collaborator in the early development of this vaccine candidate) and named as an inventor on a patent covering use of ChAdOx1-vectored vaccines (PCT/GB2012/000467) and a patent application covering this SARS-CoV-2 vaccine. TL is named as an inventor on a patent application covering this SARS-CoV-2 vaccine and was consultant to Vaccitech. PMF is a consultant to Vaccitech and has received research funding from the Brazilian Government. AJP is Chair of the UK Department of Health and Social Care's Joint Committee on Vaccination and Immunisation, but does not participate in policy advice on SARS-CoV-2 vaccines, and is a member of the WHO Strategic Advisory Group of Experts. AVSH is a cofounder of and consultant to Vaccitech and is named as an inventor on a patent covering design and use of ChAdOx1-vectored vaccines (PCT/GB2012/000467). SF is a consultant to Immunocore. GRS has received funding from Schmidt Futures and Wellcome Trust, consulting fees from GSK Vaccines Strategic Advisory Board, has patents on SARS-CoV-2 monoclonal antibodies, has leadership roles on Oxford University Council and Oxford University Hospitals NHS Foundation Trust, and holds stock in GSK. KP reports grants from the UK Medical Research Council UK Research and Innovation and National Institute of Health Research (NIHR) Vaccine Taskforce for RNA vaccine trial, COVAC1, and honoraria for Sanofi strategic advisory boards. All other authors declare no competing interests.
Figures


Comment in
-
SARS-CoV-2 vaccination in people with HIV.Lancet HIV. 2021 Aug;8(8):e455-e456. doi: 10.1016/S2352-3018(21)00128-4. Epub 2021 Jun 18. Lancet HIV. 2021. PMID: 34153265 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

