Neddylation inhibition ameliorates steatosis in NAFLD by boosting hepatic fatty acid oxidation via the DEPTOR-mTOR axis
- PMID: 34153521
- PMCID: PMC8280515
- DOI: 10.1016/j.molmet.2021.101275
Neddylation inhibition ameliorates steatosis in NAFLD by boosting hepatic fatty acid oxidation via the DEPTOR-mTOR axis
Abstract
Objective: Neddylation is a druggable and reversible ubiquitin-like post-translational modification upregulated in many diseases, including liver fibrosis, hepatocellular carcinoma, and more recently, non-alcoholic fatty liver disease (NAFLD). Herein, we propose to address the effects of neddylation inhibition and the underlying mechanisms in pre-clinical models of NAFLD.
Methods: Hepatic neddylation measured by immunohistochemical analysis and NEDD8 serum levels measured by ELISA assay were evaluated in NAFLD clinical and pre-clinical samples. The effects of neddylation inhibition by using a pharmacological small inhibitor, MLN4924, or molecular approaches were assessed in isolated mouse hepatocytes and pre-clinical mouse models of diet-induced NAFLD, male adult C57BL/6 mice, and the AlfpCre transgenic mice infected with AAV-DIO-shNedd8.
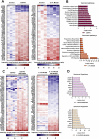
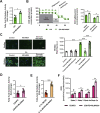
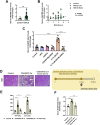
Results: Neddylation inhibition reduced lipid accumulation in oleic acid-stimulated mouse primary hepatocytes and ameliorated liver steatosis, preventing lipid peroxidation and inflammation in the mouse models of diet-induced NAFLD. Under these conditions, increased Deptor levels and the concomitant repression of mTOR signaling were associated with augmented fatty acid oxidation and reduced lipid content. Moreover, Deptor silencing in isolated mouse hepatocytes abolished the anti-steatotic effects mediated by neddylation inhibition. Finally, serum NEDD8 levels correlated with hepatic neddylation during the disease progression in the clinical and pre-clinical models CONCLUSIONS: Overall, the upregulation of Deptor, driven by neddylation inhibition, is proposed as a novel effective target and therapeutic approach to tackle NAFLD.
Keywords: Deptor; Fatty acid oxidation; MLN4924; NAFLD; Neddylation; mTOR.
Copyright © 2021 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures





References
-
- White D.L., Kanwal F., El-Serag H.B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clinical Gastroenterology and Hepatology : The Official Clinical Practice Journal of the American Gastroenterological Association. 2012;10(12):1342–1359. doi: 10.1016/j.cgh.2012.10.001. e2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

