Potential Induced Radioactivity in Materials Processed with X-ray Energy Above 5 MeV
- PMID: 34153999
- PMCID: PMC8655703
- DOI: 10.2345/0899-8205-55.s3.17
Potential Induced Radioactivity in Materials Processed with X-ray Energy Above 5 MeV
Abstract
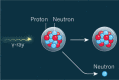
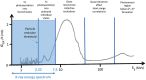
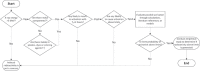
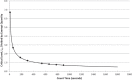
Section 5.1.2 of ANSI/AAMI/ISO 11137-1 states that "the potential for induced radioactivity in product shall be assessed." This article describes how compliance with this requirement may be achieved using qualified test methods. Materials of consideration are conceptually discussed, and results of testing conducted on products processed with a 7.5-MeV X-ray irradiation process are provided. As X-ray becomes more widely used in healthcare sterilization, having standard assessment protocols for activation coupled with a shared database of material test results will benefit manufacturers seeking to utilize this innovative technology.
© Copyright AAMI 2021. Copying, networking, and distribution prohibited.
Figures




References
-
- Smith MA. Evaluation of activation in medical products from electron beam processing at energies slightly above 10 MeV. Radiation Physics and Chemistry . 2008;77(9):1079–87.
-
- Grégoire O, Cleland MR, Mittendorfer J, et al. Radiological safety of medical devices sterilized with X-rays at 7.5 MeV. Radiation Physics and Chemistry . 2003;67(2):149–67.
-
- Smith MA. Evaluation of potential induced radioactivity in medical products as a function of electron energy in electron beam sterilization. Radiation Physics and Chemistry . 2012;81:57–63.
-
- ANSI/AAMI/ISO 11137-1:2006/(R)2015 & A1:2013 & A2:2019 Sterilization of health care products—Radiation—Part 1 Requirements for development validation and routine control of a sterilization process for medical devices . Arlington, VA: Association for the Advancement of Medical Instrumentation;
-
- International Atomic Energy Agency Natural and induced radioactivity in food . www-pub.iaea.org/MTCD/Publications/PDF/te_1287_prn.pdf Accessed March 1, 2021.
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

