Inhibition of Fibrinolysis by Streptococcal Phage LysinSM1
- PMID: 34154404
- PMCID: PMC8263008
- DOI: 10.1128/mBio.00746-21
Inhibition of Fibrinolysis by Streptococcal Phage LysinSM1
Abstract
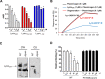
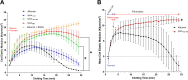
Expression of bacteriophage lysinSM1 by Streptococcus oralis strain SF100 is thought to be important for the pathogenesis of infective endocarditis, due to its ability to mediate bacterial binding to fibrinogen. To better define the lysinSM1 binding site on fibrinogen Aα, and to investigate the impact of binding on fibrinolysis, we examined the interaction of lysinSM1 with a series of recombinant fibrinogen Aα variants. These studies revealed that lysinSM1 binds the C-terminal region of fibrinogen Aα spanned by amino acid residues 534 to 610, with an affinity of equilibrium dissociation constant (KD) of 3.23 × 10-5 M. This binding site overlaps the known binding site for plasminogen, an inactive precursor of plasmin, which is a key protease responsible for degrading fibrin polymers. When tested in vitro, lysinSM1 competitively inhibited plasminogen binding to the αC region of fibrinogen Aα. It also inhibited plasminogen-mediated fibrinolysis, as measured by thromboelastography (TEG). These results indicate that lysinSM1 is a bi-functional virulence factor for streptococci, serving as both an adhesin and a plasminogen inhibitor. Thus, lysinSM1 may facilitate the attachment of bacteria to fibrinogen on the surface of damaged cardiac valves and may also inhibit plasminogen-mediated lysis of infected thrombi (vegetations) on valve surfaces. IMPORTANCE The interaction of streptococci with human fibrinogen and platelets on damaged endocardium is a central event in the pathogenesis of infective endocarditis. Streptococcus oralis can bind platelets via the interaction of bacteriophage lysinSM1 with fibrinogen on the platelet surface, and this process has been associated with increased virulence in an animal model of endocarditis. We now report that lysinSM1 binds to the αC region of the human fibrinogen Aα chain. This interaction blocks plasminogen binding to fibrinogen and inhibits fibrinolysis. In vivo, this inhibition could prevent the lysis of infected vegetations, thereby promoting bacterial persistence and virulence.
Keywords: Streptococcus mitis; fibrinogen; fibrinolysis; infective endocarditis; plasminogen; thromboelastography.
Figures





Similar articles
-
Bacteriophage lysin mediates the binding of streptococcus mitis to human platelets through interaction with fibrinogen.PLoS Pathog. 2010 Aug 12;6(8):e1001047. doi: 10.1371/journal.ppat.1001047. PLoS Pathog. 2010. PMID: 20714354 Free PMC article.
-
Characterization of the fibrinogen binding domain of bacteriophage lysin from Streptococcus mitis.Infect Immun. 2011 Sep;79(9):3518-26. doi: 10.1128/IAI.05088-11. Epub 2011 Jun 20. Infect Immun. 2011. PMID: 21690235 Free PMC article.
-
Identification and characterization of novel lysine-independent apolipoprotein(a)-binding sites in fibrin(ogen) alphaC-domains.J Biol Chem. 2003 Sep 26;278(39):37154-9. doi: 10.1074/jbc.M305154200. Epub 2003 Jul 9. J Biol Chem. 2003. PMID: 12853452
-
Molecular mechanisms of initiation of fibrinolysis by fibrin.Thromb Haemost. 2003 Mar;89(3):409-19. Thromb Haemost. 2003. PMID: 12624622 Review.
-
Fibrin-mediated plasminogen activation.Ann N Y Acad Sci. 2001;936:237-46. doi: 10.1111/j.1749-6632.2001.tb03512.x. Ann N Y Acad Sci. 2001. PMID: 11460481 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
