Progesterone-Mediated Enhancement of Hepatitis E Virus Replication in Human Liver Cells
- PMID: 34154410
- PMCID: PMC8262892
- DOI: 10.1128/mBio.01434-21
Progesterone-Mediated Enhancement of Hepatitis E Virus Replication in Human Liver Cells
Abstract
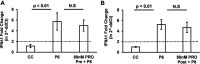
Progesterone is crucial for the maintenance of pregnancy. During pregnancy hepatitis E virus (HEV) infection is associated with increased fulminant hepatic failure and mortality rates. In this study, we determined whether progesterone modulates HEV replication and HEV-induced innate cytokine response in Huh7-S10-3 human liver cells. We first demonstrated that Huh7-S10-3 liver cells expressed SH3-domain-containing progesterone receptor membrane component (PGRMC)1/2 receptors involved in the progesterone nonclassical signaling pathway, while the classical progesterone receptor isoforms progesterone receptor-A and -B protein levels were undetectable. We showed that the genotype 3 HEV (strain P6) induced mRNA expression of type III interferon (IFN-λ1), but not other innate cytokines in Huh7-S10-3 cells. Pretreatment with progesterone at concentrations of 80 nM, 160 nM, or 480 nM, which are the physiological concentrations typically seen in the first- to third-trimester during pregnancy, significantly increased HEV replication in Huh7-S10-3 cells. However, pretreatment of cells with progesterone (80 nM) did not affect the level of HEV-induced IFN-λ1 mRNA expression. We further showed that loss of PGRMC1/2 receptors by small interfering RNA (siRNA) knockdown leads to an increase in HEV-induced IFN-λ1 expression levels at early time points via the extracellular signal-regulated kinase pathway and thus resulted in a reduced level of HEV replication. Collectively, the results indicated that progesterone-mediated modulation of HEV replication in human liver cells is plausibly through SH3-domain containing proteins such as PGRMC1/2, but not likely through immunomodulation of HEV-induced interferon response in liver cells. The results have important implications in understanding the underlying mechanisms of high mortality and fulminant hepatitis in HEV-infected pregnant women. IMPORTANCE Hepatitis E is usually a self-limiting acute disease; however, during pregnancy, a severe form of fulminant hepatic failure and high mortality rate are associated with hepatitis E virus (HEV) infection. Increased levels of progesterone and HEV RNA are observed in pregnant women with fulminant hepatic failures. Since progesterone is crucial for maintenance of pregnancy, we investigated the potential role of progesterone in HEV replication and disease pathogenesis. We demonstrated that progesterone at a concentration seen during pregnancy enhances HEV replication in human liver cells, but did not modulate HEV-induced interferon response in human liver cells. We also showed that loss of the progesterone nonclassical receptor, progesterone receptor membrane component (PGRMC)1/2, leads to a reduced level of HEV replication and an increased level of HEV-induced type III interferon (IFN-λ1) mRNA expression via the extracellular signal-regulated kinase pathway. The results from this study will aid our understanding of the underlying mechanism of pathogenesis and HEV-associated severe disease during pregnancy.
Keywords: PGRMC1/2 receptor; hepatitis E virus; pregnancy; progesterone; type III interferon; virus replication.
Figures





Similar articles
-
Modulation of SOCS3 Levels via STAT3 and Estrogen-ERαp66 Signaling during Hepatitis E Virus Replication in Hepatocellular Carcinoma Cells.J Virol. 2022 Oct 12;96(19):e0100822. doi: 10.1128/jvi.01008-22. Epub 2022 Sep 14. J Virol. 2022. PMID: 36102649 Free PMC article.
-
ISG15 Modulates Type I Interferon Signaling and the Antiviral Response during Hepatitis E Virus Replication.J Virol. 2017 Sep 12;91(19):e00621-17. doi: 10.1128/JVI.00621-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724761 Free PMC article.
-
Innate immune responses in human hepatocyte-derived cell lines alter genotype 1 hepatitis E virus replication efficiencies.Sci Rep. 2016 May 27;6:26827. doi: 10.1038/srep26827. Sci Rep. 2016. PMID: 27230536 Free PMC article.
-
Advances in Hepatitis E Virus Biology and Pathogenesis.Viruses. 2021 Feb 9;13(2):267. doi: 10.3390/v13020267. Viruses. 2021. PMID: 33572257 Free PMC article. Review.
-
Hepatitis E and Pregnancy: An Unholy Alliance Unmasked from Kashmir, India.Viruses. 2021 Jul 9;13(7):1329. doi: 10.3390/v13071329. Viruses. 2021. PMID: 34372535 Free PMC article. Review.
Cited by
-
Antiviral resistance and barrier integrity at the maternal-fetal interface restrict hepatitis E virus from crossing the placental barrier.Proc Natl Acad Sci U S A. 2025 May 6;122(18):e2501128122. doi: 10.1073/pnas.2501128122. Epub 2025 May 1. Proc Natl Acad Sci U S A. 2025. PMID: 40310464
-
Two mutations in the ORF1 of genotype 1 hepatitis E virus enhance virus replication and may associate with fulminant hepatic failure.Proc Natl Acad Sci U S A. 2022 Aug 23;119(34):e2207503119. doi: 10.1073/pnas.2207503119. Epub 2022 Aug 15. Proc Natl Acad Sci U S A. 2022. PMID: 35969750 Free PMC article.
-
Hepatitis E Virus Infection in a Pregnant Liver Transplant Recipient Leading to Chronic Infection.Transplant Direct. 2024 May 16;10(6):e1634. doi: 10.1097/TXD.0000000000001634. eCollection 2024 Jun. Transplant Direct. 2024. PMID: 38769979 Free PMC article. No abstract available.
-
Maternal hepatic immunology during pregnancy.Front Immunol. 2023 Jun 30;14:1220323. doi: 10.3389/fimmu.2023.1220323. eCollection 2023. Front Immunol. 2023. PMID: 37457700 Free PMC article. Review.
-
Role of viral hepatitis in pregnancy and its triggering mechanism.J Transl Int Med. 2024 Oct 1;12(4):344-354. doi: 10.2478/jtim-2024-0015. eCollection 2024 Sep. J Transl Int Med. 2024. PMID: 39360164 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
